Solid matter in nature can be classified into two categories: crystalline and amorphous.
A crystal is a solid with a regular geometric shape formed through the process of crystallization. In a crystal, atoms or molecules are arranged periodically and repeatedly in space according to a certain rule.
An amorphous solid, on the other hand, corresponds to a crystal with atoms or molecules arranged irregularly, without periodicity or symmetry. Glass is an example of an amorphous solid.
Solid metals and alloys are mostly crystals. The crystal structure of metals and alloys is one of the fundamental factors that determine their physical, chemical, and mechanical properties.
Iron and steel are alloy systems with iron and carbon as basic elements.
Related reading: Steel vs Iron
Within the Fe-C system, when the carbon content is below 0.02%, the material is classified as pure iron. If the carbon content exceeds 2.0%, it is referred to as pig iron, while the range between these two limits is classified as steel.
Pure iron, or wrought iron, is characterized by four crystal structures: α, β, γ, and δ. Three of these structures, namely α, β, and δ, exhibit cubic center structures, while the fourth, c, has a cubic face center structure.
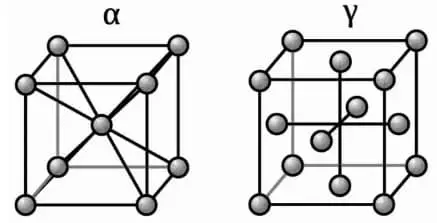
Pure elemental iron crystallizes at 1538 ℃ to form a cubic core structure known as δ-iron. As it cools to 1394 ℃, it transforms into a cubic face-centered structure called γ-iron. Further cooling to 912 ℃ results in the formation of a cubic core structure known as α-iron.
Steel has four main phases: austenite, ferrite, cementite, and martensite.
Related reading: Pig Iron vs Wrought Iron
(a) Austenite·
Austenite is a carbon interstitial compound in γ-Fe. The ratio of Fe atoms to C atoms is 27:1, meaning only one C atom is present in every 6-7 cubic face centered cells. The concentration of carbon dissolved in γ-Fe is 2.11% at 1148℃ and 0.77% at 727℃.

The characteristics of austenite are that its strength and hardness are higher compared to ferrite, while its plasticity and toughness are better. Additionally, its grain is polygonal in shape, and its grain boundary is straighter than ferrite.
(b) Ferrite·
Ferrite is a solid solution of carbon in α-Fe, with a carbon content that is close to pure iron at approximately 0.02%.
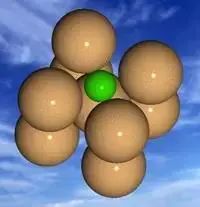
Ferrite possesses characteristics that are similar to pure iron, including low strength and hardness, and good plasticity and toughness. Its microstructure is characterized by bright polygonal grains.
(c) Cementite·
Cementite is a compound composed of iron and carbon in a 3:1 ratio, known as Fe3C. It belongs to the orthogonal crystal system and has a complex crystal structure. Each cell of cementite consists of 12 Fe atoms and 4 C atoms.
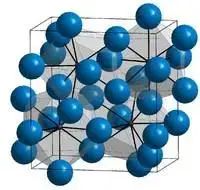
The characteristics of cementite include high hardness, poor plasticity, and toughness. Its values of δ and Akk are close to zero, and it exhibits great brittleness.
(d) Martensite·
When austenitic steel is quenched to a temperature below 150°C, it transforms into martensite, which is extremely hard. Martensite can be thought of as a supersaturated solid solution consisting of 1.6% carbon in α-Fe, and it has a tetragonal crystal structure.
There are two types of martensite: high carbon martensite (lath martensite) and low carbon martensite (lamellar martensite).
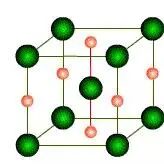
Martensite is characterized by being hard and brittle, having poor toughness, large internal stress, and being prone to cracking.
The stability of the four phases varies. Ferrite and cementite are stable crystal forms at room temperature, while austenite is stable at high temperatures.
When carbon steel is quenched, it mainly obtains martensite, which is an unstable crystal form. Alloying steels with different compositions such as Mn, Ni, and Cr can be made for different purposes.
Non-researchers in the stainless steel industry are primarily exposed to austenite, ferrite, and martensite, with cementite being less commonly encountered.
Stainless steel is a typical alloy with special properties that are achieved by adding alloy components to the basic phase.


