1. Preface
Effect of alloying elements on tempering transformation
In actual production, we can generally find some phenomena, such as:
- In order to achieve the same hardness (such as 480-610HV5) after quenching, why do carbonitriding parts require a higher tempering temperature compared to carburized parts?
- While 45 steel requires a hardness of 28-32HRC, why does 42CrMo need a higher tempering temperature?
- Why does the hardness of high-speed steel, such as SKH-9 and W6Mo5Cr4V2, increase instead of decrease after conventional high-temperature tempering? This phenomenon is attributed to the influence of alloy elements on the tempering transformation of metal parts, which will be discussed in this article.
This article provides an in-depth analysis of the topic, and we hope you enjoy reading it.
2. Effect of alloy elements on martensite decomposition
The process of decomposing martensite in alloy steel is basically similar to that of carbon steel, but the rate of decomposition differs significantly.
Experiments have demonstrated that the impact of alloy elements is particularly significant during the latter stages of martensite decomposition.
The reasons and laws of alloy elements affecting martensite decomposition can be roughly summarized as follows.
1. During the decomposition stage of martensite, the supersaturated carbon in martensite undergoes desolvation, causing precipitation and aggregation of carbide particles, resulting in a decrease in the carbon content in the matrix phase α.
The role of alloy elements is mainly to influence the decomposition process of martensite, the aggregation and growth rate of carbide particles, and the diffusion of carbon. This, in turn, affects the rate of decline of carbon concentration in phase α.
The extent of this effect varies depending on the strength of the binding force between the alloy elements and carbon.
2. Non-carbide forming elements (such as Ni and Mn) have a similar binding force with carbon to that of Fe and therefore have no significant effect on martensite decomposition.
Strong carbide forming elements (such as Cr, Mo, W, V, Ti, etc.) have a strong binding force with carbon, which increases the diffusion activation energy of carbon in martensite, hindering its diffusion and slowing down the decomposition rate of martensite.
Non-carbide forming elements such as Si and CO can dissolve into ε-FexC to stabilize it and slow down the aggregation rate of carbides, thus delaying the decomposition of martensite.
The complete desolvation temperature of supersaturated carbon in martensite during tempering of carbon steel is about 300 ℃. The addition of alloy elements can shift the complete desolvation temperature by 100-150 ℃ to a higher temperature.
In other words, alloy steel can maintain a certain saturated carbon concentration and fine carbides in phase α even when tempered at a higher temperature, thereby maintaining high hardness and strength.
Alloy elements that prevent the reduction of carbon content in phase α and the growth of carbide particles, and maintain the high hardness and strength of steel parts, are known as alloy elements that improve the tempering resistance or “backfire resistance” of steel.
3. Effect of alloy elements on Transformation of retained austenite

The transformation of retained austenite in alloy steel is similar to that of carbon steel, but alloy elements can affect the decomposition temperature and speed of retained austenite, which may alter the type and nature of the transformation.
When tempering below the MS point, residual austenite transforms into martensite.
If the MS point is high (>100 ℃), the decomposition process of martensite occurs, forming tempered martensite.
When tempering above the MS point, retained austenite may undergo three transformations:
① Isothermal transformation to bainite in the bainite formation zone;
② Isothermal transformation to pearlite in the pearlite formation zone;
③ It does not decompose during tempering heating and holding but turns into martensite in the subsequent cooling process, which is called “secondary quenching.”
Note: Is point ① related to the secondary quenching theory applied to the multiple tempering process of high-speed steel?
4. Effect of alloy elements on carbide transformation
Non-carbide forming elements such as Cu, Ni, Co, Al, Si, etc., and carbon do not form any unique types of carbides. However, they do improve the transformation from ε-FexC to θ-Fe3C as well as the conversion from cementite to other types of special carbides.
During the tempering of alloy steel, there is a redistribution of alloy elements between cementite and phase α with increasing tempering time or temperature. Carbide-forming elements continue to diffuse into cementite while non-carbide forming elements gradually enrich into phase α. This results in more stable carbides replacing the original unstable carbides, causing changes in the composition and structure of carbides.
The possible sequence of carbide transformation during tempering of alloy steel is: ε-carbide (< 150 ℃) → cementite (150-400 ℃) → cementite (alloying, 400-550 ℃) → metastable special carbide → stable special carbide (> 500 ℃). Whether special carbides can be formed in steel depends on the properties and content of the alloy elements, the carbon or nitrogen content, and the tempering temperature and time.
Typically, during the tempering process of alloy steel, cementite is transformed into stable special carbides through metastable carbides.
For example, after quenching of high Cr high carbon steel, the carbide transformation process during tempering is:
(Fe,Cr)3C→((Fe,Cr)3C)+(Cr,Fe)7C3→(Cr,Fe)7C3+(Cr,Fe)23C6→(Cr,Fe)23C6
Special carbides are also formed by these two mechanisms.
There are two types of carbide transformation processes. The first is in-situ transformation, where carbide-forming elements are initially enriched in cementite. When their concentration surpasses the solubility limit of alloy cementite, the lattice of cementite reorganizes into a unique carbide lattice. An example of this type is the transformation from (Fe, Cr) 3C to (Cr, Fe) 7C3 in low chromium steel. Increasing the tempering temperature accelerates the carbide transformation process.
The second type is nucleation and growth alone, where special carbides are directly precipitated from phase α, accompanied by the dissolution of alloy cementite. Steels containing carbide-forming elements such as V, Ti, Nb, Ta, and high Cr steels belong to this type.
For instance, 0.3% C, 2.1% V steel quenched at 1250 ℃ precipitates alloy cementite when tempered below 500 ℃, despite the low V content. Because solid solution V strongly inhibits the continuous decomposition of phase α, only about 40% of carbon precipitates in the form of cementite, and the remaining 60% is still retained in phase α.
When the tempering temperature exceeds 500 ℃, VC is directly precipitated from phase α. With further increases in tempering temperature, a significant amount of VC precipitates, and cementite dissolves. At 700 ℃, all cementites dissolve, and all carbides convert to VC.
5. Secondary hardening during tempering
In the third stage of tempering, carbon steel will continue to soften with the growth of cementite particles, as shown in Fig. 1.
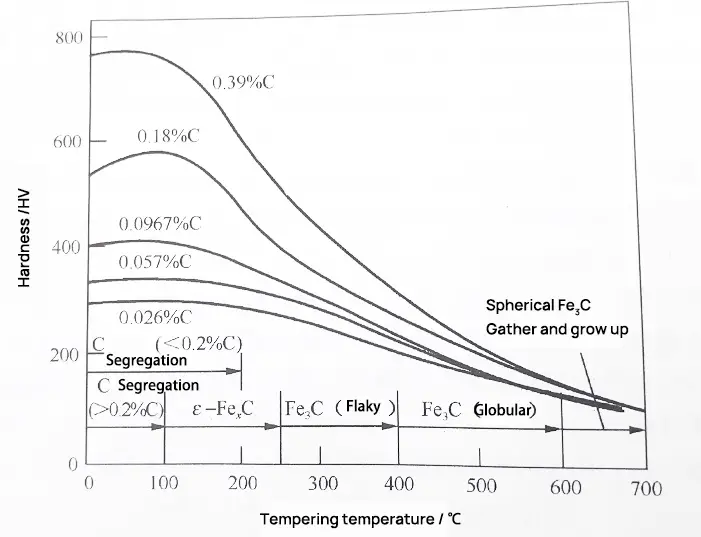
Fig. 1 hardness change of low and medium carbon steel tempered at 100-700 ℃ for 1h
However, if steel contains strong carbide-forming elements such as Mo, V, W, Ta, Nb, and Ti, the tendency to soften will be weakened, resulting in increased resistance to softening.
When martensite contains sufficient carbide-forming elements, fine special carbides precipitate during tempering above 500 ℃, causing re-hardening of the steel that has been coarsened due to the increase in tempering temperature and coarsening of θ-carbides. This phenomenon is known as secondary hardening.
In some cases, the hardness of the secondary hardening peak may be higher than that of quenching.
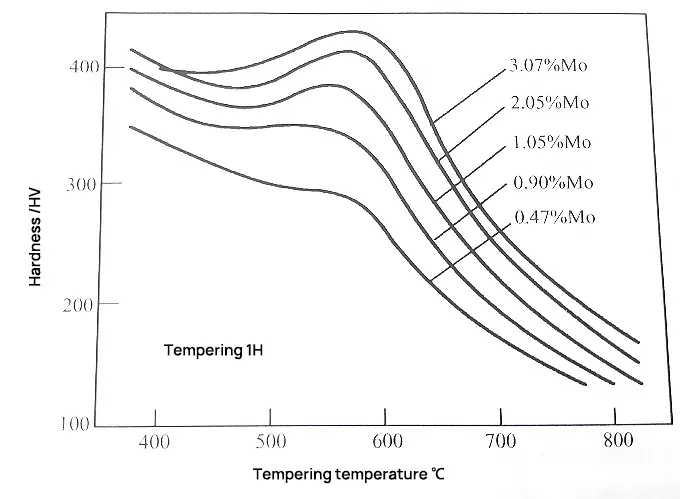
Fig. 2 Effect of tempering temperature on martensite hardness of low carbon molybdenum steel
Fig. 2 shows the effect of molybdenum content on the secondary hardening effect of low carbon (0.1%c) molybdenum steel.
The intensity of the secondary hardening effect is seen to increase with the Mo content.
Similar effects are observed with other strong carbide forming elements, such as Ti, V, W, Nb, etc.
A less distinct secondary hardening peak is seen when the Cr content is very high (more than 12%).
Carbon steel does not experience secondary hardening.
Electron microscope observations have confirmed that secondary hardening is caused by the precipitation of dispersed and fine special carbides, such as Mo2C, W2C, VC, TiC, NbC, etc.
These special carbides precipitate in the dislocation zone, often in the form of very fine needles or sheets, with a small size, and maintain a coherent relationship with phase α.
As the tempering temperature increases, the number and size of carbides gradually increase, and the lattice distortion with phase α intensifies until the hardness reaches its peak.
As the carbide grows, the dispersion decreases, the coherent relationship is destroyed, the coherent distortion disappears, and the dislocation density decreases as the temperature continues to rise, leading to a rapid decrease in hardness.
The secondary hardening effect of steel can be improved by the following ways:
- To increase the nucleation site of special carbides and enhance their dispersion, the dislocation density in steel can be increased. This can be achieved through the low temperature deformation quenching method, as shown in the figure.
- Some alloy elements can be added to the steel to slow down the diffusion of special carbide forming elements, inhibit the growth of fine carbides and delay the occurrence of over-aging of such carbides.
- For instance, the addition of CO, Al, Si, Nb, Ta, and other elements can help in maintaining coherent distortion with phase α and achieve fine dispersion of special carbides, thereby increasing the tempering stability of steel.
The alloy steel with secondary hardening effect can be selected to make the workpiece function well in a hot state. As long as the temperature used is lower than the tempering temperature (the temperature that produces the secondary hardening peak), the steel parts can maintain high hardness and strength.
6. Effect of alloy elements on the recovery and recrystallization of phase α
When alloy steel is tempered at high temperatures, it can form special carbides with fine particles that maintain a coherent relationship with phase α. This enables the steel to maintain a high carbon supersaturation of phase α and significantly delay its recovery and recrystallization. As a result, phase α remains in a highly distorted state while retaining its high hardness and strength, leading to high tempering stability.
Commonly used alloy elements in alloy steel such as Mo, W, Ti, V, Cr, Si, etc. can hinder the elimination of various distortions during tempering. They generally delay the recovery and recrystallization of phase α (increase the recrystallization temperature), as well as the aggregation and growth process of carbides, which helps to improve the tempering stability of steel.
The delaying effect of alloying elements is enhanced with an increase in their content in the steel.
When several alloying elements are added to the steel simultaneously, the interaction between them is intensified.
Alloy steel exhibits high tempering stability and maintains its high hardness and strength even at higher temperatures. This makes it suitable for tool steels such as chip cutters and hot working dies, which require red hardness and thermal strength.
7. Conclusion
This article discusses five factors that can influence the tempering transformation of alloy elements. I believe that after reading it, you will have gained valuable insights and inspiration.