
Why do some metal objects stay shiny and rust-free while others quickly lose their luster? The secret lies in the type of plating used. This article explores the differences between chrome, nickel, and zinc plating, explaining their unique properties, applications, and costs. By the end, you’ll understand how each type of plating affects durability, appearance, and corrosion resistance, helping you make informed choices for your projects.

In industrial design, the electroplating process is frequently used. Firstly, let’s understand what electroplating is. Electroplating is the process of plating a thin layer of other metals or alloys onto the surface of some metals using the principle of electrolysis.
This process uses electrolysis to attach a metal film to the surface of metal or other materials to prevent metal oxidation (such as rusting), which improves wear resistance, conductivity, light reflection, corrosion resistance (such as copper sulfate, etc.), aesthetics, and other functions.
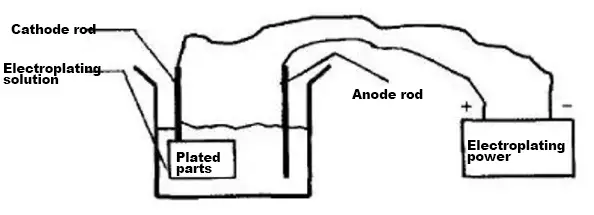
Schematic diagram of the electroplating process
Electroplating is divided into specific processes such as copper, gold, silver, chromium, nickel, and zinc plating. In the field of industrial design, especially galvanizing, nickel plating, and chromium plating are the most widely used. There must be some differences between these three methods, right?
Definition:
Zinc plating refers to a surface treatment technology that coats a layer of zinc on the surface of the metal, alloy or other materials for aesthetics and rust prevention.
Features: low cost, general corrosion protection, silvery-white color.
Applications: screws, circuit breakers and industrial supplies etc.

White zinc

Color zinc
Definition:
The method of plating a layer of nickel on metal or certain non-metals by electrolysis or chemical methods is called nickel plating.
Features: beautiful, can be used for decoration; high price; slightly complicated craftsmanship; the color is silvery white and yellow.
Application: Energy-saving lamp holder and coins etc.


Definition:
Chromium is a kind of silver-white metal with a blue tint.
The method of plating a layer of chromium on metal or some non-metals by electrolysis or chemical methods is called chromium plating.
Features: There are two types of chrome plating.
The first is for decorative purposes, with a brighter appearance, better abrasion resistance, less rustproof than galvanizing, and better than oxidation.
The second type is to increase the hardness and wear resistance etc. of metal parts, which is the functionality of the part.
Application: Bright decorative parts on home appliances, electronics and other products; faucets etc.





Chromium plating is primarily used to enhance surface hardness, improve appearance, and prevent rust.
Chromium coating boasts good chemical stability, being resistant to most alkalis, sulfides, nitric acids, and organic acids, but can be dissolved in hydrochloric acid and hot sulfuric acid.
Because chromium retains its color and reflection ability for a long time, it is better than silver and nickel. The process usually involves electroplating.
Nickel plating provides wear resistance, anti-corrosion, and rust prevention. It is typically applied in a thin layer, through either electroplating or chemical methods.
Galvanizing is focused on improving appearance and rust prevention. Zinc is a reactive metal that can react with acids, making it the least corrosion-resistant of the three and the most affordable.
In terms of cost, chromium plating is the most expensive, followed by nickel and then zinc. Note that there are two methods of plating – hanging plating, which is more costly, and barrel plating, which is more affordable.
After going on and on, some friends said “No, it’s still the same, silly and unclear.” So I must admit that I might get confused if I keep talking about it. In this case, it may be easier to simply distinguish the different types of plating by their color.

Extended Knowledge:
The production of electroplating is mainly used to combat heavy metal pollution in wastewater and sewage. The government has strict controls on the expansion of the electroplating industry and has been reducing it year by year.
In China, the main processes for electroplating are zinc, copper, nickel, and chromium plating, with 50% being zinc plating and 30% each for copper, chromium, and nickel plating.
If the goal is to prevent rust, then zinc or cadmium plating can be utilized. If the focus is to prevent wear and tear, then nickel or chromium plating is the best option.







