Ever wondered why connecting copper and aluminum wires is problematic? This article explains the risks associated with connecting these two metals due to their differing electrochemical properties, which can lead to corrosion, increased resistance, and even fire hazards. Learn how using proper transition clamps or tinned wires can prevent these issues and ensure safe electrical connections. By reading, you’ll understand the critical steps to avoid potential electrical failures in your projects.

Copper and aluminum have distinct chemical and physical properties, making direct connections between them inadvisable. The primary issue lies in their differing electrochemical properties, which can lead to significant problems when the two metals are connected directly.
When copper and aluminum come into contact with an electrolyte (such as water containing impurities like carbon dioxide), they form a galvanic cell. In this cell, aluminum acts as the anode (negative electrode) because it has a higher tendency to lose electrons, while copper acts as the cathode (positive electrode) due to its lower tendency to lose electrons. This creates an electromotive force of approximately 1.69V between the two metals, resulting in a small current that corrodes the aluminum wire. This phenomenon, known as electrochemical corrosion, leads to poor contact and increased contact resistance.
The increased contact resistance at the joint causes it to heat up when a current passes through. This heating accelerates the corrosion process, creating a vicious cycle that can eventually lead to the joint burning out and potentially causing a fire. Therefore, it is crucial to avoid direct connections between copper and aluminum wires to prevent these safety hazards.
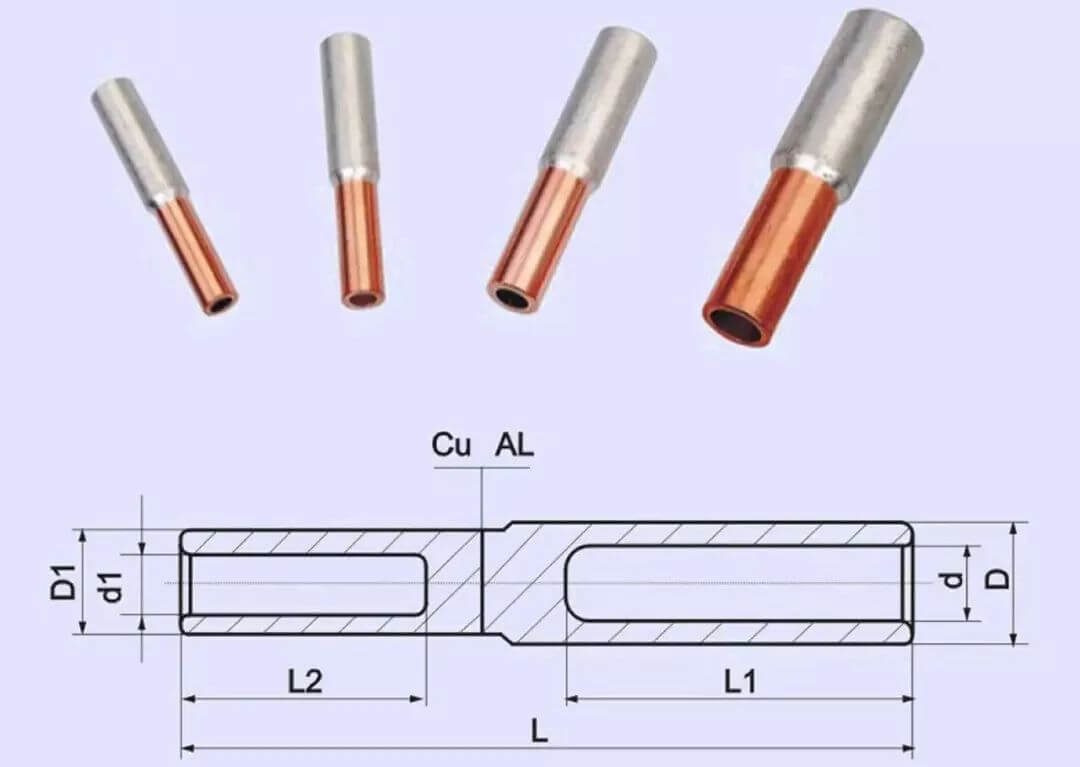
In situations where connecting copper and aluminum is unavoidable, specific methods and materials can be used to mitigate the risks:

Advantages:
Disadvantages:
Advantages:
Disadvantages:
Directly connecting copper and aluminum wires is not recommended due to the risk of electrochemical corrosion and the potential for increased contact resistance, heating, and fire hazards. If a connection between the two metals is necessary, using transition clamps, tinned copper wire, or copper-aluminum transition joints is essential to ensure safety and reliability. Despite the higher cost, copper wire is generally a better investment due to its superior electrical properties and stability.