Have you ever wondered why stainless steel, known for its durability, can still rust? This article explores the surprising truth behind stainless steel corrosion, revealing the types and causes of rust. Learn how to protect your equipment and ensure longevity in various environments.
Stainless steel is known for its resistance to rust. The primary alloy element in stainless steel is chromium (Cr). Only when the chromium content reaches a certain level can it exhibit corrosion resistance. Typically, the chromium content in stainless steel must be at least 10.5%.
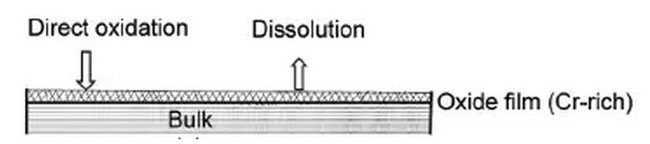
Illustration of bell coating on stainless steel surface
The mechanism behind the corrosion resistance of stainless steel is known as the passive film theory, which states that an ultra-thin, solid, fine, and stable chromium-rich passive film forms on its surface, blocking the infiltration and oxidation of oxygen atoms, thereby providing protection against corrosion.
While many people believe that “stainless steel does not rust,” this statement is incorrect. Under specific conditions, stainless steel can still corrode.
Related reading: Why Stainless Steel Rust & How to Prevent It From Rusting?
It is important to note that by understanding the various types of corrosion that can affect stainless steel, measures can be taken to minimize losses when faced with stainless steel corrosion.
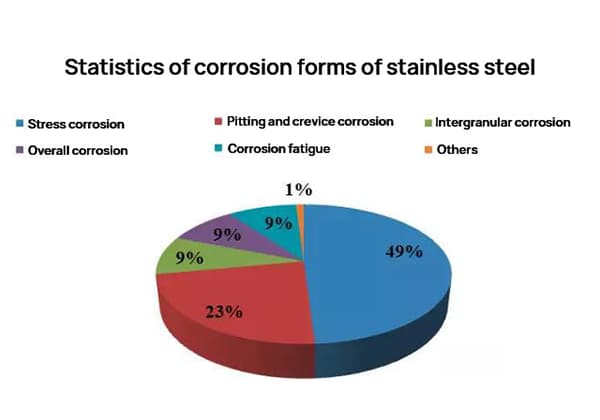
Most of the corrosion damage experienced by stainless steel is localized corrosion, with the most common types being intergranular corrosion (9%), pitting corrosion (23%), and stress corrosion (49%).
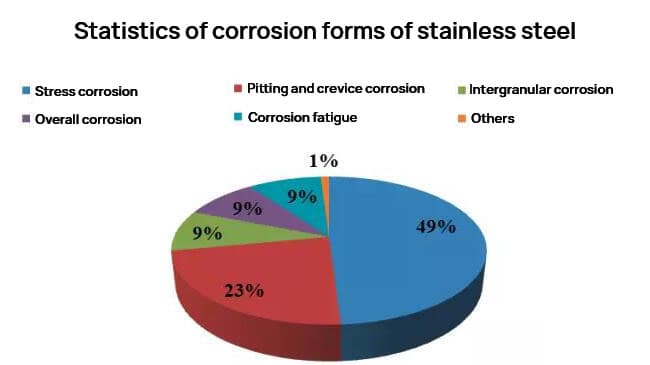
Stainless steel can often provide adequate corrosion resistance in many industrial applications. Based on practical experience, apart from mechanical failures, the corrosion of stainless steel is primarily characterized by local corrosion (such as stress corrosion cracking, pitting corrosion, intergranular corrosion, corrosion fatigue, and crevice corrosion). These forms of local corrosion account for over half of the failure cases. In reality, many of these failures can be prevented through proper material selection.
Stress corrosion cracking (SCC) is a general term that refers to the combined failure of stressed alloys caused by the spread of severe cracks in corrosive environments. Although it typically has a brittle fracture appearance, SCC can still occur in materials with high toughness. The necessary conditions for stress corrosion cracking are the presence of tensile stress (whether residual or external, or both) and a specific type of corrosive medium. The formation and growth of the crack is generally perpendicular to the direction of tensile stress, and the stress level required for SCC is much lower than what is needed for fracture in the absence of a corrosive medium.
Microscopically, cracks that run through grains are referred to as transgranular cracks, while cracks that run along grain boundaries are called intergranular cracks. When stress corrosion cracking reaches a certain depth (at which point the stress in the material under load reaches its fracture stress in air), the material will break just as it would with normal cracks (in ductile materials, usually through the aggregation of microscopic defects). The section of a part that fails due to stress corrosion cracking will therefore contain both the characteristic area of stress corrosion cracking and the “tough-rich” area associated with the aggregation of micro defects.
It is a type of localized corrosion that results in corrosion.
The intergranular boundaries are the boundaries between different crystallographic orientations of disordered and staggered intergranular elements. As a result, they are prone to segregation of various solute elements or precipitation of metal compounds (such as carbides and δ phases) in steel. In certain corrosive environments, it is not uncommon for the grain boundaries to corrode first, leading to a type of corrosion known as intergranular corrosion. This type of corrosion can occur in most metals and alloys in specific corrosive media.
Crevice corrosion is a type of localized corrosion that occurs in areas where a solution is stagnant or in shielded surfaces. This type of corrosion can occur at joints between metal and metal or metal and non-metal, such as at the contact points with bell studs, bolts, gaskets, valve seats, loose surface deposits, and marine organisms.
General corrosion refers to the corrosion that occurs on the entire surface of an alloy in a relatively uniform manner. This type of corrosion can cause the material to gradually become thinner and, in severe cases, may result in the material becoming unusable due to corrosion. Stainless steel may experience general corrosion in strong acids and alkalis. The failure issues caused by general corrosion are not as concerning because this type of corrosion can usually be predicted through simple immersion testing or by consulting corrosion literature.