What exactly is intergranular corrosion in stainless steel, and why is it so critical to control it? This insidious form of corrosion attacks along the grain boundaries of the metal, often leading to catastrophic failures without visible warning signs. In this article, we’ll explore the mechanisms behind intergranular corrosion, the environmental conditions that exacerbate it, and the best practices for preventing it. You’ll gain a comprehensive understanding of how to safeguard stainless steel structures, ensuring their longevity and reliability.
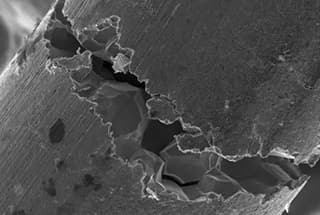
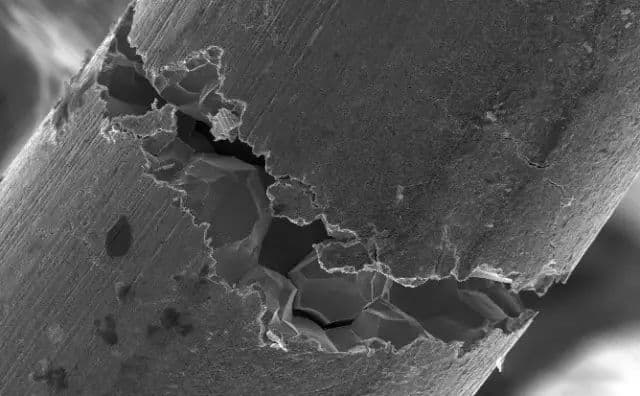
The unified technical regulations generally require that austenitic stainless steel vessels used in environments that may cause intergranular corrosion must undergo solid solution or stabilizing treatment after welding. This requirement is reasonable.
However, even if the designer includes this requirement in the technical specifications of the drawing, it is often difficult for the manufacturer to meet the ideal standards due to challenges in controlling the heat treatment process parameters and other unforeseen difficulties. In reality, most stainless steel equipment in use today is used without undergoing post-welding heat treatment.
This raises the question: what is the mechanism of intergranular corrosion, which is the most common form of corrosion in austenitic stainless steel? What are the environmental conditions that can lead to intergranular corrosion? What are the main methods for preventing and controlling intergranular corrosion? Are heat treatments necessary for austenitic stainless steel vessels used in environments that may cause intergranular corrosion after welding?
This article will explore these questions by referencing relevant standards, specifications, and monographs, and by presenting personal opinions based on production experience.
Intergranular corrosion is a type of localized corrosion that occurs along the grain boundaries or in the vicinity of the grain boundaries of a metal or alloy. This corrosion is characterized by minimal corrosion within the grains and significant corrosion along the grain boundaries, which weakens the bond between the grains.
If intergranular corrosion is severe, it can reduce the strength and ductility of the metal, causing it to fail under normal loads. The two main theories behind intergranular corrosion are the theory of low chromium content and the theory of selective dissolution of impurities at the grain boundaries.
The intergranular corrosion of commonly used austenitic stainless steel in oxidizing or weakly oxidizing environments is usually caused by improper heating during processing or use. Improper heating refers to heating or slowly cooling the steel through the temperature range of 450-850°C, which makes it vulnerable to intergranular corrosion. This temperature range is therefore considered dangerous for austenitic stainless steel.
Austenitic stainless steel undergoes solution treatment before it leaves the factory. The solution treatment involves heating the steel to 1050-1150°C and then rapidly cooling it to create a homogeneous solid solution. Austenitic steel contains a small amount of carbon, and its solid solubility decreases with decreasing temperature. For example, the solid solubility of carbon in 0Cr18Ni9Ti is about 0.2% at 1100°C and about 0.02% at 500-700°C.
The carbon in solution-treated steel is therefore supersaturated. When the steel is heated or cooled through 450-850°C, the carbon can precipitate from the austenite and distribute along the grain boundaries in the form of (Fe, Cr) 23C6. The chromium content of (Fe, Cr) 23C6 is much higher than that of the austenitic matrix, and its precipitation consumes a large amount of chromium near the grain boundaries, which cannot be replenished in a timely manner through diffusion. The slow diffusion of chromium causes the chromium content near the grain boundaries to fall below the 12% Cr limit required for passivation, creating a chromium-poor region and damaging the passive state.
The grain itself, however, still maintains a passive state with a high potential. The grain and the grain boundary form a micro galvanic battery, with a large cathode and a small anode, leading to corrosion in the grain boundary region.
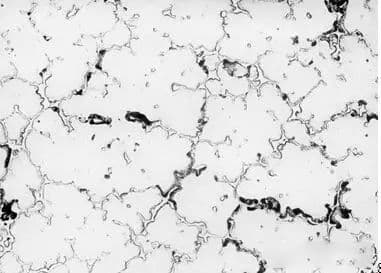
In production practice, we have observed that austenitic stainless steel can also experience intergranular corrosion in strong oxidizing media (such as concentrated nitric acid), but the nature of the corrosion is different from that in oxidizing or weak oxidizing media. Intergranular corrosion in strong oxidizing media usually occurs in solid solution-treated steel, but does not occur in sensitized steel.
If the impurities, such as phosphorus or silicon, reach 100ppm or 1000-2000ppm respectively in the solid solution, they will segregate along the grain boundaries. These impurities will dissolve under the action of strong oxidizing media, causing intergranular corrosion.
When the steel is sensitized, the formation of (MP) 23C6 with phosphorus or the first segregation of carbon eliminates or reduces the segregation of impurities at the grain boundaries, thus eliminating or weakening the steel’s sensitivity to intergranular corrosion.
These two theories on the mechanism of intergranular corrosion apply to the structural state of a particular alloy and medium, and are not mutually exclusive, but rather complementary. In production practice, the majority of intergranular corrosion cases occur in weak oxidizing or oxidizing media, and can therefore be explained by the low chromium theory.
There are two main types of media that cause intergranular corrosion in austenitic stainless steel. The first type is oxidizing or weak oxidizing media, and the second type is strong oxidizing media, such as concentrated nitric acid. The first type of media is more common.
Here is a list of common medium environments that cause intergranular corrosion in austenitic stainless steel:
The “Corrosion Data Chart” prepared by G A. Nelson lists the common mediums that cause intergranular corrosion in austenitic stainless steel:
When using austenitic stainless steel in an environment that may cause intergranular corrosion, the intergranular corrosion tendency test must be conducted according to the GB4334.1 to GB4334 test methods for intergranular corrosion of stainless steel. The selection and qualification requirements for the test methods for intergranular corrosion tendency of austenitic stainless steel must meet the following criteria:
(1) Austenitic stainless steel and special stainless steel for concentrated nitric acid used in nitric acid with a temperature of 60°C or higher and a concentration of 5% or higher must be tested according to the GB4334.3 test method for 65% nitric acid corrosion of stainless steel. The average corrosion rate over five cycles or three cycles must not exceed 0.6g/m2h (or equivalent to 0.6mm/a). The sample can be in use or sensitized.
(2) Chromium nickel austenitic stainless steel (such as 0Cr18Ni10Ti, 0Cr18Ni9, 00Cr19Ni10, and similar steels): General requirements: according to the GB4334.5 sulfuric acid copper sulfate corrosion test method for stainless steel, there must be no intergranular corrosion cracks on the surface of the sample after the bending test. Higher requirements: the average corrosion rate must not exceed 1.1g/m2h according to the GB4334.2 sulfuric acid ferric sulfate corrosion test method for stainless steel.
(3) Molybdenum-containing austenitic stainless steel (such as 0Cr18Ni12Mo2Ti, 00Cr17Ni14Mo2, and similar steels): General requirements: according to the GB4334.5 sulfuric acid copper sulfate corrosion test method for stainless steel, there must be no intergranular corrosion cracks on the surface of the sample after the bending test. Higher requirements: the corrosion ratio must not exceed 1.5 according to the GB4334.4 nitric acid hydrofluoric acid corrosion test method for stainless steel. The average corrosion rate must not exceed 1.1g/m2h according to the GB4334.2 sulfuric acid ferric sulfate corrosion test method for stainless steel.
(4) If the medium has special requirements, intergranular corrosion tests other than those specified above can be conducted, and the corresponding qualification requirements must be specified.
According to the mechanism of corrosion, the following measures can be taken to prevent and control intergranular corrosion in austenitic stainless steel:
(1) Utilizing ultra low carbon stainless steel can help lower the carbon content to below 0.03%.
For instance, 00Cr17Ni14Mo2 can be chosen to prevent the formation of (Fe, Cr) 23C6 in the steel and the occurrence of a chromium-poor zone, thereby avoiding intergranular corrosion.
Typically, for components that have low strength, low stress, and good plasticity, 0Cr18Ni9 can be selected for its cost-effectiveness.
(2) Stabilized stainless steel refers to the stainless steel that contains titanium and niobium.
During the production of the steel, a specific amount of titanium and niobium is added, and these elements have a strong affinity with carbon, forming tic or NBC within the steel.
Additionally, the solid solubility of tic or NBC is much smaller than that of (Fe, Cr) 23C6 and is almost insoluble in austenite at the solid solution temperature.
This way, even if (Fe, Cr) 23C6 is not precipitated on the grain boundary when the sensitization temperature is reached, the likelihood of intergranular corrosion in austenitic stainless steel is greatly reduced.
For example, steels like 1Cr18Ni9Ti and 1Cr18Ni9Nb can function within a temperature range of 500-700°C without experiencing intergranular corrosion.
(3) When welding austenitic stainless steel with an electric arc, the temperature of the arc pool can reach as high as 1300°C, and the temperature on both sides of the weld decreases with increasing distance, creating a sensitization temperature zone.
It is ideal to heat and cool the austenitic stainless steel as slowly as possible within the sensitization temperature range.
In the case of intergranular corrosion tendencies, the unstable stainless steel should be heated to 1000-1120°C for 1-2 minutes per millimeter and then quenched.
For stabilized stainless steel, heating to 950-1050°C is recommended.
After undergoing solution treatment, it is important to prevent the steel from being heated at the sensitization temperature, as this may cause chromium carbide to be precipitated along the grain boundary once again.
(4) Choosing the correct welding method is important to reduce the sensitivity of welded joints to intergranular corrosion. If the operation remains unchanged or the welding material is too thick, a longer welding time increases the chances of staying within the sensitized temperature zone.
To minimize the sensitivity of the welded joints, it is necessary to minimize the input of line energy during welding.
Generally speaking, argon arc welding has a lower input line energy compared to electric arc welding, making it a better choice for welding and repair.
For welding parts, it is recommended to use ultra low carbon stainless steel or stainless steel with stabilizing elements like titanium and niobium. Additionally, using ultra low carbon welding rods or niobium-containing welding rods is recommended.
When using argon arc welding, to avoid overheating of the welding joint, the operation should be quick and the base metal on both sides of the weld should be cooled rapidly after welding to minimize the time spent within the sensitization temperature range.

Post-weld heat treatment is not always a priority in the weld area.
Typically, a solid solution treatment is carried out at a temperature range of 1100-1150°C for a certain duration and then quenched. The cooling within the range of 925-540°C should be completed within three minutes, followed by rapid cooling to below 425°C.
For stabilized treatment, the workpiece should be air-cooled after being held at a temperature range of 850-880°C for several hours.
The effectiveness of post-weld heat treatment is highly dependent on key process parameters such as furnace temperature, temperature rise rate, temperature difference among various parts of the workpiece during the temperature rise, furnace atmosphere, holding time, temperature difference among various parts during the heat preservation, cooling rate, and furnace temperature.
For austenitic stainless steel vessels that may cause intergranular corrosion, solution treatment or stabilized treatment of general parts can be performed. However, post-weld heat treatment of the entire vessel (usually a heat exchanger) presents many difficulties.
This type of treatment is not a local post-weld heat treatment but rather a post-weld heat treatment of the entire welded parts or vessel.
Due to the complex structure and shape of most chemical vessels, such as the commonly used shell and tube heat exchanger, controlling the key process parameters for post-weld solid solution or stabilized treatment of the entire shell and tube heat exchanger is nearly impossible, let alone ensuring the quality of the post-weld heat treatment.
In many cases, this treatment may even prove to be counterproductive, not only failing to improve the weld structure but also deteriorating the base metal structure unnecessarily.
Therefore, more than 90% of austenitic stainless steel chemical vessels used in intergranular corrosion environments are still used in their post-weld state rather than undergoing post-weld heat treatment.
Chromium nickel austenitic stainless steel is the most widely used corrosion-resistant material, and intergranular corrosion is the most common form of failure in chromium nickel austenitic stainless steel vessels.
Intergranular corrosion significantly weakens the bond between grains and, in severe cases, can completely eliminate the mechanical strength. The surface of the stainless steel that has undergone this type of corrosion remains bright but can be easily broken into fine particles with gentle tapping.
Intergranular corrosion is difficult to detect, which can lead to sudden equipment damage and should be taken seriously.
Chromium nickel austenitic stainless steel vessels are typically formed through welding, and the two sides of the welded joint are intergranular corrosion-sensitized areas, which are more susceptible to corrosion damage compared to the base metal.
Post-weld heat treatment can improve the resistance to intergranular corrosion in the weld zone to the same level as the base metal. This is the ultimate goal of post-weld heat treatment.
However, in practice, there are many factors to consider, such as the complex overall structure and shape of the weldment, which makes it difficult to guarantee the process parameters of post-weld heat treatment.
As a result, most in-service chromium nickel austenitic stainless steels are used after welding.
Whether the weld zone of a chromium nickel austenitic stainless steel vessel used for intergranular corrosion resistance undergoes solid solution treatment or stabilized treatment cannot be generalized. The structural shape of the vessel must be analyzed to determine if the heat treatment can be effectively carried out. Otherwise, even if post-weld heat treatment is required, it may have adverse effects, not only failing to achieve the desired result but also affecting the base metal structure.
To enhance the intergranular corrosion resistance of chromium nickel austenitic stainless steel vessels, it is necessary to select ultra-low carbon stainless steel and stabilized stainless steel based on the specific corrosion environment and mechanism, choose the correct welding method during welding, and properly combine the previously mentioned prevention and control measures to achieve good results.
Reliance on solid solution or stabilize treatment after welding is not sufficient.