How can a material be both a solution and a challenge in industrial applications? Ultrapure ferritic stainless steel, with its remarkable corrosion resistance and thermal conductivity, is essential in various industries. However, its high chromium content introduces brittleness at certain temperatures. This article explores the benefits and complexities of using this steel, detailing its properties, common issues like brittleness, and factors affecting its performance. By reading, you’ll understand how ultrapure ferritic stainless steel is shaping modern manufacturing while posing unique production challenges.
Ferritic stainless steel refers to a type of stainless steel that has a chromium (Cr) mass fraction of between 12% to 30%. It can be further divided into low Cr, medium Cr, and high Cr, depending on the Cr mass fraction.
The corrosion resistance of ferritic stainless steel is proportional to the Cr mass fraction. The higher the Cr mass fraction, the greater the resistance to corrosion. However, to enhance the overall properties and reduce the negative impact of Cr carbide and nitride precipitation on the mechanical properties and corrosion resistance, the trend in ferritic stainless steel development is towards lower carbon (C) and nitrogen (N) levels.
Ultrapure ferritic stainless steel is a subcategory of ferritic stainless steel that has very low levels of C and N (generally no more than 0.015% combined) and medium to high Cr mass fractions. This type of stainless steel is popular due to its good corrosion resistance, thermal conductivity, seismic resistance, processing performance, and affordability compared to copper, copper alloys, and titanium materials. It is widely used in various industries, including the automotive industry, kitchen and household appliances, construction, and petrochemical industries.
However, there are also several challenges in producing ultrapure ferritic stainless steel. Due to its high Cr mass fraction and the presence of other alloying elements like molybdenum (Mo) and manganese (Mn), it is difficult to avoid the inherent problems of high Cr ferritic stainless steel, such as σ-phase brittleness, 475 ℃ brittleness, and high-temperature brittleness.
The production personnel are therefore aware of the potential harm of these brittleness issues and have found that they are primarily caused by precipitation of σ-phase, χ-phase, α’-phase, Laves phase, and the mass fraction of the Cr element.
This article provides an in-depth examination of the main characteristics and influencing factors of σ-phase brittleness, 475 ℃ brittleness, and high-temperature brittleness in ultrapure ferritic stainless steel. It also analyzes the effects of these brittleness issues on the mechanical properties and corrosion resistance of ultrapure ferritic stainless steel, serving as a reference for producers and users.
Ultra-pure ferritic stainless steel contains various alloy elements and is prone to the precipitation of different intermetallic compounds during hot working, mainly carbon and nitrogen compounds of Cr, Nb, and Ti, as well as intermetallic compounds of phases σ, χ, Laves, and α.
The characteristics of phases σ, χ, Laves, and α’ are presented in Table 1.
Table 1 Characteristics of Intermetallic Compounds in Ultrapure Ferritic Stainless Steel
| Precipitated phase | Structure | Configuration and composition | Precipitation condition | Characteristic |
| σ mutually | Body centered tetragonal (bct) D8b, 30 atoms/unit cell | AB or AxBy, FeCrFeCrMo | w(Cr)=25%~30%,600-1050℃ | Hard, brittle, rich in Cr |
| X phase | Body centered cubic (bcc) A12, 30 atoms/unit cell | α- Mn, Fe36Cr12Mo10 or (Fe, Ni) 36Cr18Mo4 | w(Mo)=15%~25%,600-900℃ | Hard, brittle, rich in Cr and Mo |
| Laves phase | Close packed hexagonal (hcp) C14 or C36 | AB2, Fe2Ti or Fe2Nb or Fe2Mo | 650-750℃ | Hard |
| α’ mutually | Body centered cubic (bcc) | Fe Cr, rich in cr | w(Cr)>15%,371-550℃(475℃) | Hard, brittle, rich in Cr |
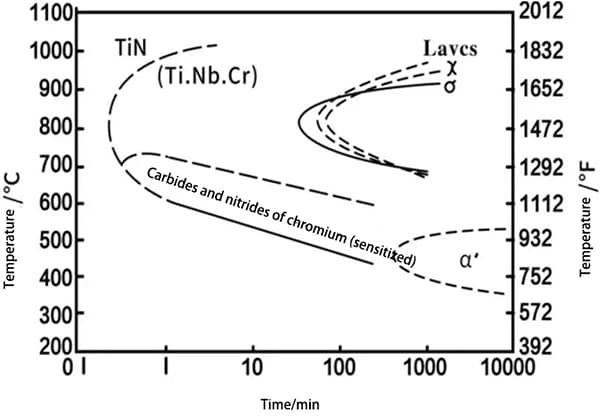
The precipitation “C” curves for the σ, χ, and Laves phases of some typical ultra-pure ferritic stainless steels are shown in Figures 1 and 2.
Due to variations in alloy composition, the most sensitive temperature range for precipitation of these phases is between 800 and 850°C.
For the 00Cr25Ni4Mo4NbTi (Monit) alloy, the σ and χ phases precipitate relatively quickly, while the Laves phase is most easily precipitated at 650°C and takes more time to form.
Regardless of the type of brittle precipitate, excessive precipitation will make the steel brittle, resulting in a sharp decline in impact properties.
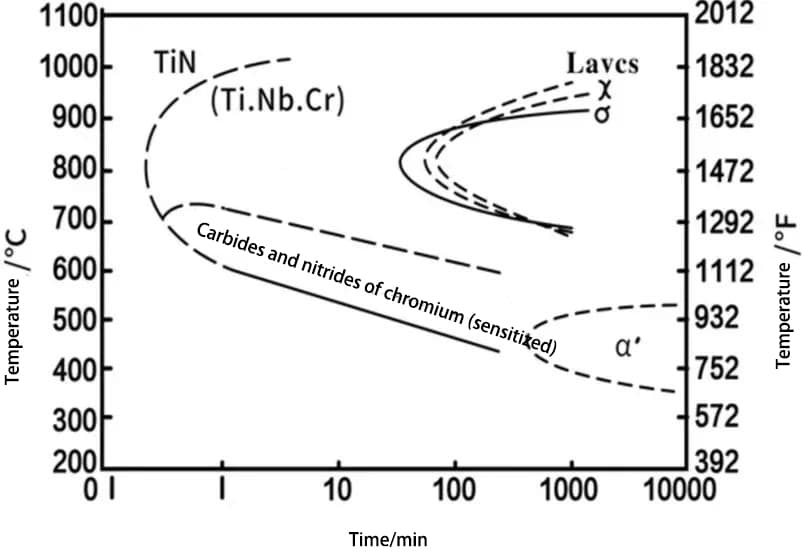
Fig. 1 26% Gr – (1%~4%) Mo – (0~4%) Ni Ferritic Stainless Steel

Fig. 2 TTP Diagram of 00Cr25Ni4Mo4TiNb (Monit) Ferritic Stainless Steel (after solid solution at 1000 ℃)
The generation of σ-phase brittleness is primarily caused by the precipitation of σ-phase and χ-phase. The Laves phase has a similar precipitation temperature, so it is included in the discussion.
1.1.1 σ mutually
The σ-phase is a size-factor compound with an AB or AxBy configuration and a body-centered tetragonal structure. In ferritic stainless steels, the σ-phases are primarily composed of FeCr or FeCrMo.
Under conditions where the Cr content (w(Cr)) is between 25% and 30% and the precipitation temperature is between 600 and 1050 ℃, the formation of σ-phase is facilitated. The formed phase enriches the Cr element, as shown in Figure 3.
The σ-phase is non-magnetic and has high hardness, with a Rockwell hardness (HRC) value of up to 68. During the precipitation process, a “volume effect” occurs, which decreases the plasticity of the steel.

Fig. 3 Structure and composition of o phase of 447 ferritic stainless steel under EDX linear analysis
The precipitation of the σ phase can seriously weaken stainless steel, diminishing its properties such as corrosion resistance, impact toughness, and mechanical properties.
The formation of σ phase occurs in two stages: nucleation and growth. Nucleation typically starts at the grain boundary of α/α’ and expands from there into the matrix.
Once the σ phase reaches a certain size, it precipitates from within the grain.
1.1.2 Phase χ
Ultra-pure ferritic stainless steel will not only form the σ phase, but also the σ phase, when it contains a certain amount of Mo element.
The structure of the χ phase is body-centered cubic and of the α-Mn type.
In ferritic stainless steel, the χ phase is primarily composed of Fe36Cr12Mo10 or (Fe, Ni)36Cr18Mo4.
Typically, it forms under conditions where the Mo content (w) is between 15% and 25% and the temperature is between 600 and 900℃.
The toughness of the steel decreases significantly when the χ phase is formed.
It was found that compared to the σ phase, Cr and Mo are enriched more rapidly in the χ phase and precipitate more quickly in the χ phase than in the σ phase.
Generally, the χ phase has the same structure as the ferrite matrix.
Due to its low nucleation potential barrier, nucleation is relatively straightforward, and the χ phase usually precipitates earlier than the σ phase, as shown in Fig. 4.

Fig. 4 χ Phase Precipitated from 26Cr Ferritic Stainless Steel Aged at 800 ℃ for 5min
When the χ phase begins to form, there will be a significant enrichment of Cr and Mo in the χ phase, leading to a decrease in the content of Cr and Mo. This decrease is not enough to nucleate the σ phase, making the formation of the σ phase difficult at the initial stage.
Additionally, the χ phase is metastable and its stability decreases with aging time. As the χ phase decomposes, it will provide enough Cr and Mo to nucleate the σ phase, eventually leading to its transformation into a stable σ phase.
Both the χ phase and the σ phase will result in a reduction of the Cr content around the precipitation phase through precipitation, forming a Cr-poor zone and decreasing its corrosion resistance.
1.1.3 Laves phase
The Laves phase is a size factor compound with an AB2 configuration and a hexagonal structure, as depicted in Figure 5.
In ferritic stainless steel, the Laves phase is typically composed of Fe2Ti, Fe2Nb, or Fe2Mo.
The Laves phase in ferritic stainless steel is enriched with Si elements, which play a crucial role in maintaining its stability.
The precipitation temperature of the Laves phase ranges from 650-750℃, depending on the composition of the alloy.

Fig. 5 Laves Phase Precipitated from 27Gr-4Mo-2Ni Ferritic Stainless Steel after Aging at 1050 ℃ for 1h
Andrade T et al. found that after aging at 850°C for 30 minutes, the ultra-pure ferritic stainless steel with the model DIN 1.4575 shows precipitation of the Laves phase at the grain boundary, which remains unchanged in size due to the presence of both Laves and σ phase precipitates. The growth rate of the σ phase is faster, preventing some of the Laves phase from growing.
It was discovered that the 11Cr-0.2Ti-0.4Nb ferritic stainless steel, when aged at 800°C for 24-28 hours, exhibits a large number of Laves phase precipitates that increase slowly over time. However, when the aging time reaches 96 hours, the Laves phase transformation becomes coarse and the number decreases, with no precipitation of the σ phase observed.
The ferritic stainless steel with a chromium mass fraction greater than 12% will experience a significant increase in hardness and strength, accompanied by a sharp decrease in plasticity and impact toughness after prolonged exposure to temperatures between 340 to 516℃. This is mainly due to the brittleness that occurs in ferritic stainless steel at 475℃.
The most sensitive temperature for this property change is 475 ℃.
The precipitation of α ‘phase is the main reason for the 475 ℃ brittleness of ferritic stainless steel.
α ‘phase is a Cr rich brittle phase with a body centered tetragonal structure.
In ferritic stainless steel, α ‘phase is easy to form under the condition that w (Cr) is greater than 15% and precipitation temperature is 371~550 ℃.
α’ phase is a Fe Cr alloy, with Cr content ranging from 61% to 83% and Fe content ranging from 17.5% to 37%.
The literature indicates that when the Cr content in steel is below 12% by mass, there will be no precipitation of the α’ phase, thus avoiding the formation of 475℃ brittleness.
Additionally, the precipitation of the α’ phase during dissolution is a reversible process.
When the steel is reheated to above 516℃ and then rapidly cooled to room temperature, the α’ phase will dissolve back into the matrix and the brittleness at 475℃ will not reoccur.
When the Cr content in ferritic stainless steel is between 14% to 30%, rapid cooling after heating the steel above 950℃ can result in decreased elongation, impact toughness, and resistance to intergranular corrosion. This is mainly due to the high-temperature brittleness of ferrite.
The main cause of high-temperature brittleness is the precipitation of Cr-carbon and Cr-nitrogen compounds. Furthermore, during the welding process, the precipitation of Laves phase can occur when the welding temperature exceeds 950℃, impacting the overall properties of the steel.
This vulnerability also exists in ultra-pure ferritic stainless steel, which is even more sensitive to high-temperature brittleness due to its high Cr and Mo content.
To reduce the risk of high-temperature brittleness, the C and N content can be reduced and stabilizing elements can be added.
In welding, high-temperature brittleness can result in significant damage to the steel. This is because C and N elements precipitate at the grain boundary during welding and react with Cr and Mo, forming Cr and Mo-rich carbon and nitrides that gradually move towards the grain boundary.
Additionally, the precipitation of Laves phase at 950℃ during welding can lead to precipitates at dislocations, grain boundaries, or within grains, inhibiting the movement of crystal dislocations and grain boundaries. This results in the local arrangement of atoms becoming more regular, increasing the strength of the steel but reducing its plasticity and toughness.
The following elements – Cr, Mo, Ti, Nb, W, and Cu – in ultra-pure ferritic stainless steel have an impact on the formation of brittle precipitates.
An increased concentration of the Cr element in ferritic stainless steel leads to improved passivation, resulting in better resistance to surface oxidation, and improved resistance to pitting, crevice corrosion, and intergranular corrosion.
However, a higher mass fraction of Cr also leads to a faster formation of brittle phases in the ferritic stainless steel. The formation and precipitation speed of α’ and σ phases are also influenced by the mass fraction of Cr, with a higher mass fraction leading to a faster precipitation speed. This precipitation phase reduces the toughness of the steel and significantly increases its brittle transition temperature.
Mo is the second most important element in ferritic stainless steel. When its mass fraction reaches a certain level, the precipitation amount of σ and χ phases in ferritic stainless steel increases significantly.
Research by Moura et al. found that the addition of Mo in 25Cr-7Mo ferritic stainless steel reduced the maximum precipitation temperature of the α’ phase, bringing it down from 475°C to around 400°C and increasing the number of α’ phases.
Kaneko et al. found that Mo contributes to the quicker accumulation of Cr in the passivation film, thus improving the stability of the film and strengthening the corrosion resistance of Cr in steel.
Ma et al. found that annealing 30Cr steel at 1020°C resulted in the precipitation of the Laves phase, which is mainly composed of Fe, Cr, Mo, Si, and Nb. The mass fraction of Nb and Mo in the Laves phase was higher compared to the base metal. The X-ray energy spectrum analysis of the Laves phase of 30Cr steel annealed at 1020°C is shown in Fig. 6.
It was observed that an increased Mo content in 30Cr ultra-pure ferritic stainless steel accelerates the precipitation of the Laves phase. Literature suggests that an increased Mo content leads to the precipitation of Mo-rich χ-phase in 26Cr stainless steel after aging, and with extended aging time, part of the Laves phase transforms into σ-phase.

Fig. 6 X-ray Energy Spectrum Analysis (EDS) of Laves Phase of 30Cr Steel after 1020 ℃ Annealing
(a) EDS analysis of base metal; (b) EDS Analysis of Laves Phase
The addition of stable elements, such as Nb and Ti, to steel combined with C and N results in the precipitation of phases such as TiN, NbC, and Fe2Nb. These phases are distributed both within the grain interior and at grain boundaries, which slows the formation of Cr carbides and nitrides, thereby enhancing the intergranular corrosion resistance of ferritic stainless steels.
Anttila et al. studied the impact of incorporating Ti and Nb into the welds of 430 ferritic stainless steel. They found that when the welding temperature reached 950 ℃, Laves phase formation was facilitated, leading to the embrittlement of the welded joints and a decrease in their impact toughness.
Similarly, Naghavi and other researchers discovered that the solubility of Nb in the matrix of ferritic stainless steel decreases with increasing temperature during high temperature aging, causing the coarsening of Laves phase and a decrease in the tensile strength of the steel.
The inclusion of W in 444 ferritic stainless steel was found to significantly improve its high temperature tensile strength when aged at 1000 ℃. However, as the mass fraction of W increases, the Laves phase coarsens, weakening the precipitation strengthening effect and reducing the high temperature tensile strength.
The addition of Cu to ferritic stainless steel precipitates a Cu-rich phase, which significantly improves the corrosion resistance of 430 Cu. Fe-Cu binary alloys and Fe-Cu-Ni ternary alloys containing Cu can improve the strength and toughness of the steel.
The Cu-rich phase mainly precipitates at 650 ℃ and 750 ℃, and during the initial aging stage, it remains spherical. As the aging temperature and time increase, it gradually transforms into an elliptical and rod-shaped form, as depicted in Figure 7.
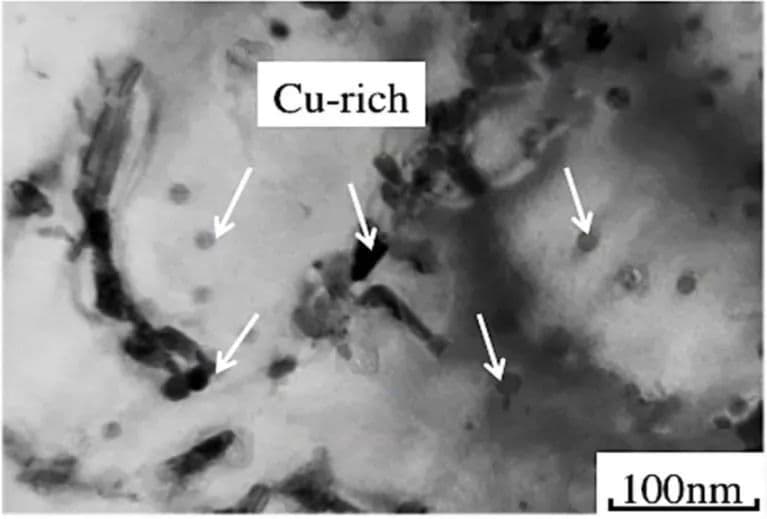
Fig. 7 Morphology of Cu rich phase in 17Cr-0.86Si-1.2Cu-0.5Nb ferritic stainless steel aged at 750 °C for 1h
Rare earth elements (REs) are highly reactive chemically and adding the appropriate amount of REs can improve the properties of steel.
The TEM test results of precipitates in 27Cr ferritic stainless steel are presented in Fig. 9.
Without REs, the precipitated phases in ferritic stainless steel are more complex. As illustrated in Fig. 8(a), secondary phases precipitate at the grain boundaries and form chains in the ferrite matrix, consisting mainly of σ phase, M23C6, M6C, and a small amount of M2N and χ phases.
However, after adding REs, the chain precipitated phases decrease and are often present in single forms in the matrix, mainly as σ phase. Furthermore, the precipitation of carbon and nitride decreases, as shown in Fig. 8(b).
The optimal RE mass fraction in ultra-pure ferritic stainless steel was found to be 0.106%, which enhances the strengthening properties. At this concentration, REs refine the grain structure, increase the impact energy, and change the impact fracture mechanism from brittle to tough.
Additionally, REs reduce the mass fraction of S in steel, reducing the source of pitting corrosion and improving the pitting corrosion resistance.

Fig. 8 TEM Results of Precipitated Phase of 27Cr Ferritic Stainless Steel
(a) Bright field image of 0% RE sample; (b) Bright field image of 0.106% RE sample
Different aging treatments can have varying impacts on the formation of brittle precipitates in materials.
When pure ferritic stainless steel forms brittle precipitates, it can result in a decline in its mechanical properties, impact resistance, corrosion resistance, and overall performance.
Aging treatment can help to improve the material’s structure and increase its plasticity, as well as effectively reducing the formation of precipitates and limiting their negative effects on the steel.
LU HH et al. discovered that when 27Cr-4Mo-2Ni ferritic stainless steel is aged at temperatures ranging from 600 to 800°C, the main precipitates formed are χ phase, Laves phase, and σ phase.
The morphologies and distributions of these phases in the 27Cr-4Mo-2Ni ferritic stainless steel aged at different temperatures are depicted in Figure 9.
The presence of these precipitates can decrease the impact toughness, tensile strength, and plasticity of the material while increasing its hardness.
After aging at temperatures between 600 to 800°C, the χ phase mainly precipitates along the grain boundaries. The Laves phase is precipitated within the grain when the material is aged at 700°C, while the σ phase generally forms at the grain boundaries after aging at 750°C.
At this point, the Laves phase partially dissolves into the matrix, providing Cr and Mo atoms for the growth of the σ phase. This coarsening of the grain can lead to brittle fracture in the steel.

Fig. 9 Morphology and Distribution of x Phase, Laves Phase and o Phase of 27Cr-4Mo-2Ni Ferritic Stainless Steel Aged at Different Temperatures
(a) Aging at 650 ℃ for 4h; (b) Aging at 700 ℃ for 4h; (c) Aging at 750 ℃ for 2h; (d) Aging at 800 ℃ for 4h.
Zhang Jingjing discovered that when SUS444 ultra-pure ferritic stainless steel was aged at 850℃ for 10 minutes, TiN transformed into a composite structure of TiN/NbC/Nb poor phase. The bonding strength between the composite structure and the matrix is high, which significantly improves the impact toughness.
Luo Yi and colleagues found that when 446 ultra-pure ferritic stainless steel was aged at 800℃, phase σ precipitated after 0.5 hours and increased with aging time, forming a network-like structure. Simultaneously, microcracks appeared in phase σ and its high quantity reduced the steel’s toughness.
Ma Li and others annealed 26% Cr ultra-pure ferritic stainless steel and found that there were mainly three precipitates: TiN, NbC, and χ. The harmful χ phase seriously led to brittleness in the steel. With increasing annealing temperature up to 1020℃, the χ phase gradually decreased to a negligible amount. Thus, to eliminate the χ phase, a high annealing temperature is necessary.
For the high Cr ferritic stainless steel 27.4Cr-3.8Mo-2.1Ni, QUHP and others found that after aging at 950℃ for 0.5 hours, σ and Laves phases precipitated, improving the steel’s hardness but decreasing its ductility. These harmful phases could be dissolved into the matrix after solution treatment at 1100℃ for 0.5 hours.
Wu Min and colleagues found that when 441 hot-rolled plate was annealed at 900-950℃, a large number of Laves phases precipitated. As shown in Figure 10, there are two precipitated phases: (1) the primary phase, which is a composite structure of (Ti, Nb) (C, N) with a size of approximately 5 μm and (2) the Laves phase, which is small, numerous, dense, and uniformly distributed in grain boundaries, subgrain boundaries, and grains. Increasing the annealing temperature to 1000-1050℃ effectively eliminated Laves phase, but a small amount of Nb (C, N) phase precipitated.

Fig. 10 Laves Phase Morphology of 441 Ferritic Stainless Steel Hot Rolled Plate after Different Annealing Temperatures
(a) Laves phase appearance after annealing at 900 ℃; (b) Laves phase appearance after annealing at 950 ℃.
The research shows that high levels of Cr and Mo and a certain amount of Nb in the microstructure can easily lead to the formation of brittle intermetallics, such as the (Fe Cr Mo) type σ phase, the (Fe Cr Mo) type χ phase, and the Fe2Nb type Laves phase. These brittle intermetallics result in a significant decrease in the plastic toughness and an increase in the hardness of ultra-pure ferritic stainless steel.
German scholar Saha R and colleagues found that the low solubility of the element C causes ferritic stainless steel to precipitate high-hardness (Ti, Nb) C during high-temperature cooling, and the dispersed (Ti, Nb) C improves the strength and hardness of the steel.
The research also found that the two-phase particles Cr23C6 and Cr2N in the alloy have a strong impact on the mechanical properties, particularly toughness and ductility, leading to a reduction in toughness and ductility and a higher risk of fracture.
Typical precipitation of the α’ phase leads to a depletion of Cr in the ferrite matrix, reducing the corrosion resistance and toughness of the steel and increasing its hardness.
It was discovered that when 444 ferritic stainless steel is aged at temperatures between 400-475 ℃, the precipitation of the α’ phase leads to an increase in hardness, but after aging for more than 500 hours at 475 ℃, its toughness drops sharply.
Figure 11 shows the hardness of 441 ultra-pure ferritic stainless steel and the energy absorbed by fracture after aging.

Fig. 11 Change of hardness and fracture absorbed energy of 441 ultra pure ferritic stainless steel with time after aging at 400 ℃ and 450 ℃
(a) Hardness changes with aging time; (b) The energy absorbed by fracture varies with aging time.
Luo Yi and colleagues discovered that the tensile strength of 446 ultra-pure ferritic stainless steel can be improved to some extent when the network structure of phase σ has not formed after aging treatment.
However, when phase σ precipitation forms a network structure, the tensile strength and elongation of the material decrease significantly, as illustrated in Figure 12.
Moreover, regardless of whether a network structure forms, the precipitation of phase σ causes severe harm to the material’s impact property, leading to a decrease in its impact property and failing to meet certain requirements for steel.

Fig. 12 Change of tensile strength and elongation of 446 ultra pure ferritic stainless steel with time after aging at 800 ℃
The precipitation of the Laves phase in ultra-pure ferritic stainless steel has both positive and negative impacts.
According to literature, with prolonged aging time, the Fe2Nb phase will start to precipitate in the steel, causing a decrease in its toughness and high temperature strength.
However, adding Si and Nb elements to the Laves phase precipitation leads to an increase in the creep resistance and high temperature strength of the steel. The presence of W in the Laves phase also helps improve the high temperature tensile strength of the steel.
As illustrated in Fig. 13, compared to non-W type 444 ferritic stainless steel, the tensile strength is significantly improved when the W mass fraction is between 0.5% and 1%.
When aging at 900 ℃, the tensile strength decreases slightly with increasing aging time, but eventually stabilizes. At 1000 ℃, the tensile strength may decrease significantly, but the initial tensile strength remains higher than that of the non-W steel.

Fig. 13 Variation of High Temperature Tensile Strength of 444 Ferritic Stainless Steel with Aging Time at 900C and 1000 ° C
(a)900℃; (b)1000 ℃。
The Laves phase will precipitate from 441 ferritic stainless steel during aging at 850 ℃ and will grow rapidly. When it forms a network structure along the grain boundary, it reduces the plasticity and impact toughness of the steel. As the number of grain boundaries decreases and the grain size becomes larger, the precipitation rate decreases.
The mechanical properties of 19Cr-2Mo Nb Ti ferritic stainless steel at different aging temperatures are displayed in Fig. 14. During the aging process of the steel at temperatures between 850 ℃ and 1050 ℃, the (FeCrSi)2(MoNb) and (Fe, Cr)2(Nb, Ti) type Laves phases will transform into (Nb, Ti)(C, N) precipitates. The mass fraction of Nb in the solution will increase due to the dissolution and coarsening of the precipitates, leading to a reduction in its tensile strength.
However, after aging treatment at 950 ℃, the homogeneity of the recrystallized grains is improved and the elongation increases sharply, reaching 37.3%. It then gradually stabilizes at 32.6%.

Fig. 14 Mechanical Properties of 19Cr-2Mo-Nb-Ti Ferritic Stainless Steel at Different Aging Temperatures
It has been found that the precipitation of the brittle phase will negatively impact the corrosion resistance of steel.
Moreover, according to literature, the high Cr mass fraction of 27.4Cr-3.8Mo ultra-pure ferritic stainless steel leads to the formation of phases σ and χ after aging at 950°C for 0.5 hours, resulting in a decrease in pitting resistance.
However, aging at 1100°C for 0.5 hours causes phases σ and χ to gradually disappear and the pitting resistance to recover. The change in pitting potential is illustrated in Figure 15.

Fig. 15 Pitting Potential of 24.7Cr-3.4Mo and 27.4cr-3.8Mo Stainless Steel
The content of chromium (Cr) and molybdenum (Mo) in stainless steel plays a crucial role in its corrosion resistance. When the Cr mass fraction exceeds 25% and the temperature is between 700-800°C, the precipitation of σ and χ phases will occur, leading to a decrease in corrosion resistance.
Additionally, Cr easily combines with carbon (C) and nitrogen (N) elements, causing precipitation at the grain boundary or within the grain. This leads to the formation of Cr-rich carbon and nitride, reducing the Cr mass fraction and corrosion resistance. The precipitates also harm the passivation film, causing it to lose its uniformity and stability, thereby affecting the steel’s corrosion resistance.
Welded joints in corrosive environments are prone to intergranular, pitting, crevice, and other types of local corrosion. Researchers such as Huang Zhitao have found that increasing the Mo mass fraction in high-purity ferritic stainless steel in chloride environments can delay the precipitation of M23C6 (where M is Fe, Cr, and Mo) and improve pitting corrosion resistance.
Zhang Henghua et al. discovered that adding a certain amount of Mo to 26Cr ultra-pure ferritic stainless steel can enrich the Cr in the passivation film and enhance its stability, thereby improving the material’s pitting corrosion resistance. Tong Lihua et al. found that adding niobium (Nb) and titanium (Ti) to ultra-pure ferritic stainless steel can effectively prevent the precipitation of Cr carbon and nitrogen compounds and enhance its intergranular corrosion resistance.
However, other studies have shown that high levels of Ti and N in 15Cr ultra-pure ferritic stainless steel can lead to the formation of TiN, which accelerates the growth of pitting corrosion and negatively impacts the material’s corrosion resistance. Wen Guojun and colleagues found that aging 430Ti ferritic stainless steel at 475°C for 0-100 hours leads to an increase in hardness, α’ and α phases, and a significant decrease in corrosion resistance, as shown in Figure 16.

Fig. 16 Corrosion Resistance of 430Ti Ferritic Stainless Steel
In conclusion, the higher the Cr mass fraction in ultra-pure ferritic stainless steel, the more likely it is to produce precipitates that severely reduce its corrosion resistance. Adding appropriate amounts of niobium (Nb), titanium (Ti), and molybdenum (Mo) can improve the steel’s corrosion resistance, however, the formation of TiN from Ti has a negative impact on the pitting corrosion resistance of the steel.
The main characteristics and factors that influence the σ-phase brittleness, 475°C brittleness, and high temperature brittleness of ultra-pure ferritic stainless steel are analyzed in this paper. The following conclusions are drawn:
(1) The brittleness of the σ-phase in ultra-pure ferritic stainless steel is due to the precipitation of the σ-phase and the χ-phase, which are rich in chromium and molybdenum elements. The brittleness at 475°C is due to the precipitation of the chromium-rich α’-phase. The high temperature brittleness is caused by the precipitation of carbon and chromium nitride.
(2) The alloying elements, rare earth elements (RE), and aging treatments in ultra-pure ferritic stainless steel have a certain impact on the precipitated phases, which can to some extent inhibit the generation of σ-phase brittleness, 475°C brittleness, and high temperature brittleness.
The following are the specific impacts:
① The precipitation of α ‘, σ , χ , and Laves phases increases when the content of Cr and Mo increases. In ultra-pure ferritic stainless steel, adding stabilizing elements can reduce or eliminate high-temperature brittleness in thin sections. High-temperature brittleness can be avoided by avoiding high temperatures during heat treatment. The addition of Ti and Nb can also delay the precipitation of σ phase, reducing its brittleness. However, the addition of Ti and Nb leads to the generation of Laves phase, and a high content of Nb can cause coarsening of Laves phase.
② The addition of RE reduces the precipitation of carbon and nitride in σ and Cr phases, reducing the brittleness of σ phase and high-temperature brittleness, and improving the mechanical properties and pitting resistance of steel.
③ Different aging treatments have varying effects on precipitates. Precipitates may differ slightly based on the Cr content. When aging at 600-800 ℃, a small amount of σ , χ , and Laves phases precipitate. At 600 ℃, α ‘phase re-dissolves in the matrix, and brittleness disappears at 475 ℃. A large number of σ , χ , and Laves phases precipitate when aging at 850-950 ℃. When aging at 1000-1100 ℃, the precipitation of σ , χ , and Laves phases is reduced or even disappears. The brittleness of σ phase can be eliminated by aging treatment above 1000 ℃.
(3) The precipitation of secondary phases such as α’, σ, χ, and Laves in ultra-pure ferritic stainless steel can have a significant impact on its mechanical and corrosion properties. The precipitation of these phases reduces the steel’s toughness and plasticity, increases its strength and hardness, and affects its corrosion resistance.
The addition of Si and W elements to the Laves phase enhances its high-temperature strength and tensile strength. Furthermore, the addition of Cu elements results in Cu-rich phase precipitation, which improves the steel’s toughness.
Domestic Ni resources are scarce, and excessive consumption can lead to a shortage, which will severely impact the stainless steel industry.
Ultra-pure ferritic stainless steel, as a resource-saving steel, has high comprehensive performance and low comprehensive cost, making it an inevitable choice for the domestic stainless steel industry to promote 400 series stainless steel with low nickel content.
Ultra-pure ferritic stainless steel has gradually replaced some austenitic stainless steel in industries such as automotive, household appliances, and elevators. It has also been successfully used in the construction of large building roofs, such as airports and stadiums.
The market for ultra-pure ferritic stainless steel is expected to grow in the future, with a large market scale and broad prospects.
In the future, it is crucial to focus on the brittleness of ultra-pure ferritic stainless steel. To ensure good mechanical properties and corrosion resistance, it is necessary to effectively restrain the generation of σ-phase brittleness, 475℃ brittleness, and high-temperature brittleness during production and use. By doing so, the advantages of “resource-saving” can be fully utilized, leading to greater progress and development in the stainless steel industry.