Why do some aluminum alloys corrode more easily, and what can we do about it? This article explores the causes and types of corrosion in 6000 series aluminum alloys, focusing on intergranular corrosion. Readers will learn about various corrosion forms, why 6000 series alloys are vulnerable, and practical measures to prevent these issues, ensuring better performance and longevity for aluminum structures.
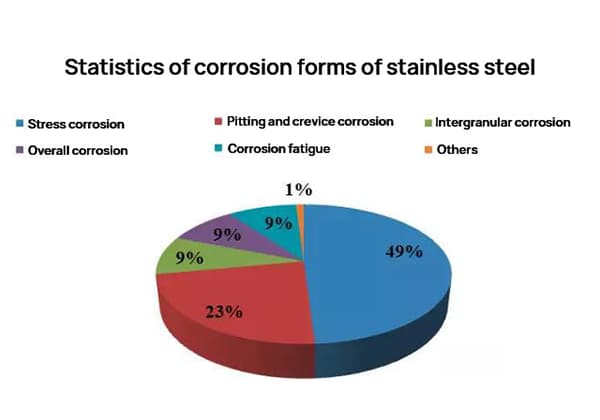
According to conventional methods of estimation, the direct economic loss caused by corrosion in China is approximately 3% of GDP annually, with the steel consumed by corrosion accounting for about one-third of the annual output, of which about one-tenth is not recyclable.
The corrosion resistance of aluminum and aluminum alloys is significantly higher than that of steel, leading to much smaller corrosion losses. However, regardless of the metal material or its level of corrosion resistance, corrosion loss will always occur to some degree during use.
The annual corrosion loss of aluminum is estimated to be around 0.5% of the aluminum output for that year. The types of corrosion that occur in aluminum and aluminum alloys include pitting corrosion, intergranular corrosion, stress corrosion cracking, and layered corrosion.
The 6000 series aluminum alloys have the highest output among wrought aluminum alloys. Although their corrosion resistance is not as good as that of 1000, 3000, and 5000 series aluminum alloys, it is still significantly higher than that of 2000 and 7000 series aluminum alloys.
The 6000 series alloys have a relatively high tendency for intergranular corrosion, so it is important to evaluate their sensitivity to intergranular corrosion for critical structures.
The appearance of corrosion in aluminum can be divided into two types: comprehensive corrosion and local corrosion.
Comprehensive corrosion, also known as uniform corrosion, refers to the loss that occurs evenly across the surface of the material when it comes into contact with the environment. An example of uniform corrosion in aluminum is the corrosion that occurs in an alkaline solution, such as during alkali washing.
The result of uniform corrosion is that the aluminum surface becomes thinner at a relatively consistent rate, leading to a reduction in mass. However, it should be noted that absolute uniform corrosion does not exist, and the thinning of thickness may vary in different areas.
Local corrosion refers to the corrosion that is limited to specific areas or parts of the structure. This type of corrosion can be further divided into several categories, including:
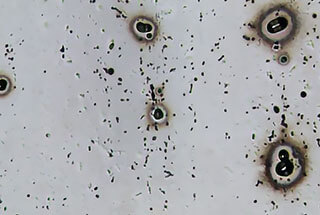
Pitting corrosion occurs in isolated areas of the metal surface and results in small cavities or pits that can grow and eventually lead to perforation.
If the diameter of the pit opening is smaller than its depth, it is referred to as pitting corrosion. If the diameter of the pit opening is larger than its depth, it is referred to as pit erosion.
There is no clear boundary between pitting corrosion and pit erosion.

A typical example of pitting corrosion in aluminum is in an aqueous solution containing chloride.
Pitting corrosion is the most common type of corrosion in aluminum and is caused by differences in potential between certain areas of aluminum and the aluminum matrix, or by the presence of impurities with a different potential from that of the aluminum matrix.
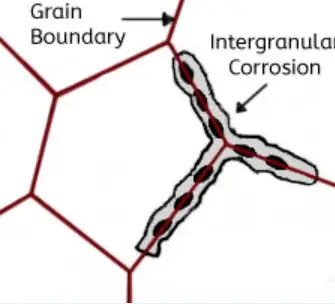
This type of corrosion selectively affects the grain boundaries of the metal or alloy, without causing significant erosion of the grains or crystals. It results in a sharp reduction of the material’s mechanical properties, leading to structural damage or failures.
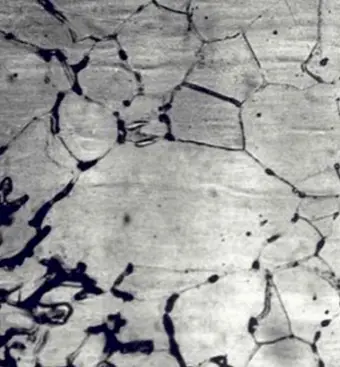
Intergranular corrosion occurs when certain conditions result in increased activity at the grain boundaries, such as impurities at the grain boundaries or fluctuations in the concentration of alloy elements at the grain boundaries.
In other words, there must be a thin layer at the grain boundary that is electronegatively charged relative to the rest of the aluminum, making it more susceptible to corrosion. This type of corrosion can occur in high-purity aluminum in hydrochloric acid and high-temperature water. Alloys such as AI Cu, AI Mg Si, Al Mg, and Al Zn Mg are particularly sensitive to intergranular corrosion.
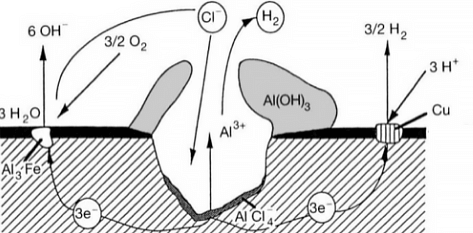
Galvanic corrosion is a common form of corrosion in aluminum.
When two metals with different levels of activity, such as aluminum (the anode) and a less active metal, come into contact in the same environment or are connected through a conductor, a galvanic couple is formed and current flows, causing galvanic corrosion. This type of corrosion is also known as bimetallic corrosion or contact corrosion.
Aluminum has a very negative natural potential, and when it comes into contact with other metals, it is always the anode, which accelerates the corrosion process. Almost all aluminum and aluminum alloys are susceptible to galvanic corrosion.
The greater the potential difference between the two metals in contact, the more severe the galvanic corrosion. It is important to note that the surface area ratio is critical in galvanic corrosion, and the most unfavorable combination is a large cathode and small anode.
Crevice corrosion occurs when two same or different metals come into contact, or when a metal comes into contact with a non-metal, creating a gap. Corrosion occurs at or near the gap due to the lack of oxygen in the area, which creates a concentration cell.
Crevice corrosion is not dependent on the type of alloy and can occur even in highly corrosion-resistant alloys. The acidic environment at the top of the gap is the driving force behind the corrosion and is a form of corrosion under sediment (scale).
An example of crevice corrosion under scale is the corrosion that occurs under mortar on the surface of 6063 alloy building aluminum profiles.
Joint corrosion can be caused by sludge, scale, and impurities on the metal surface of flange connections, fastening surfaces, lap surfaces, weld pores, under rust layers, and sink layers.
Stress corrosion cracking is a type of corrosion that occurs when tensile stress and a specific corrosion medium exist simultaneously. The stress can be external or residual stress within the metal, which can be caused by factors such as deformation during processing and manufacturing, severe temperature changes during quenching, or volume changes resulting from changes in internal structure.
The residual stress can also be caused by processes such as riveting, bolt fastening, press fit, and cold shrinkage fit.
When the tensile stress on the metal surface reaches the yield strength Rp0.2, stress corrosion cracking will occur.
The 2000 and 7000 series aluminum alloys can produce residual stress during quenching, which should be eliminated through pre-stretching prior to aging treatment to avoid deformation or the introduction of stress into aircraft parts during processing.
Layered corrosion, also known as delamination, spalling, or denudation for short, is a specific form of corrosion that occurs in 2000 series, 5000 series, 6000 series, and 7000 series alloys. It is commonly seen in extruded materials and once it occurs, it can peel off layer by layer like mica.

Filiform corrosion is a type of creeping corrosion that can develop under aluminum paint films or other coatings, but it is not found under anodic oxide films. This type of corrosion is commonly found in aircraft aluminum structural parts and building or structural aluminum parts.
The occurrence of filiform corrosion is influenced by factors such as material composition, pretreatment before coating, and environmental factors such as temperature, humidity, and chloride levels.
The 6000 series of aluminum alloys, which can be strengthened through heat treatment, are the most commonly used wrought aluminum alloys today. They are Al Mg Si and Al Mg Si Cu alloys and in 2018, 126 of the 706 registered alloys in the Aluminum Association, Inc. were 6000 series alloys, accounting for 18%.
These alloys are widely used in the construction, structural, and transportation industries due to their good processing formability, moderate strength, and excellent corrosion resistance. However, if the alloy composition ratio is not appropriate, the heat treatment parameters are not correctly selected, or the processing and forming is improper, intergranular corrosion can occur in chlorine-containing environments.
Most intergranular corrosion occurs in alloys with a small amount of copper and high Si/Mg ratio. Generally, the copper content in most copper-containing alloys is no more than 0.4%, with only four alloys, such as 6013, 6113, 6056, and 6156 having a copper content as high as 1.1%. Copper is added to Al Mg Si alloys to improve the mechanical properties of the alloy.
High-resolution scanning transmission electron microscopy reveals that copper-rich segregation layers and cathodic q-phase precipitates are often found in alloys with intergranular corrosion sensitivity. The q-phase is a quaternary intermetallic phase with the molecular formula Cu2Mg8Si5Al4, which precipitates along the grain boundary, causing anodic dissolution of the adjacent solid solution and forming a precipitate-free zone.
There are two common methods for determining the intergranular corrosion sensitivity of aluminum alloys: field testing and accelerated immersion testing. In accelerated testing, a potassium chloride solution containing hydrochloric acid (ISO 11846 method B) or a potassium chloride solution with hydrogen peroxide (ASTM G110) is often used to accelerate the corrosion process.
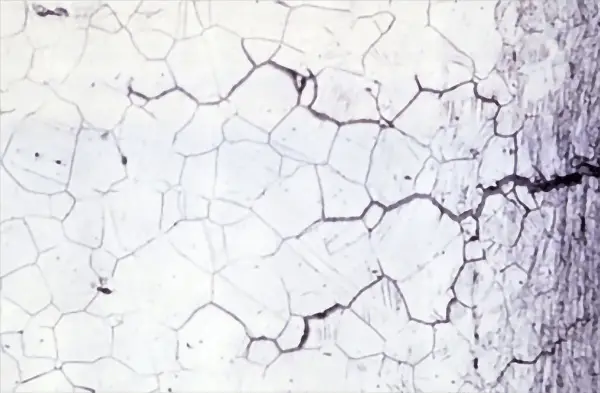
After the test, the cross-section of the sample is examined through metallography or the loss of mechanical properties is measured. The results of the ISO 11846 accelerated test are highly consistent with the field test results in a marine atmosphere.
However, during the accelerated test, nearly all grain boundaries close to the sample surface undergo severe corrosion (uniform intergranular corrosion), while in the field test, the sample surface only corrodes in limited areas (local corrosion). Despite this difference, the accelerated test is still a standard method for accurately judging the presence of grain boundary corrosion in materials.
The automotive industry often determines if a 6000 series aluminum alloy has intergranular corrosion according to the ISO 11846 method B standard. This involves immersing a small sample (surface area less than 20 cm2) in a room temperature acidic sodium chloride solution (pH = 1) for 24 hours and then conducting a metallographic inspection to determine the type of corrosion (pitting or intergranular).
It is essential to determine the extent of surface corrosion damage and the maximum corrosion depth. Recent studies indicate that making some significant modifications to the test conditions will not significantly affect the reproducibility of the results.
The standard specifies that the ratio of electrolyte volume to sample surface area must not be less than 5ml/cm2, or it will significantly impact the intergranular corrosion rate. For the surface of the sample to corrode, there must be cathodic reaction (hydrogen precipitation and oxygen reduction), and the pH value of the test solution must increase over time, leading to a decrease in electrolyte corrosion.
Out of the 8 series of wrought aluminum alloys, the 6000 series alloy is a type of Al Mg Si (Cu, Zn) alloy and is highly susceptible to intergranular corrosion. This series has a high intergranular corrosion sensitivity.
To test the intergranular corrosion tendency of the 6000 series alloy, the most effective method is to conduct alkaline etching as per ISO 11846 standard and then perform decontamination treatment using concentrated nitric acid solution. However, the results may be affected by etching in NaOH solution with a temperature of 50-60℃ and mass fraction of 5-10% for 2-5 minutes.
A more effective alternative to alkali etching is to use nitric acid/hydrofluoric acid solution, which can effectively remove aluminum from iron-rich protoplast spots on the surface. Aluminum particles can accelerate the corrosion of aluminum alloys in chloride solutions as they are local micro cathodes and the source of intergranular corrosion.
The corrosion of the alloy in nitric acid/fluoride solution is slower compared to the corrosion in alkali solution.

The 6000 series alloy is not only a widely used, highly outputted and diverse deformed aluminum alloy, but it is also one of the deformed alloys with high sensitivity to intergranular corrosion. Nevertheless, intergranular corrosion can be prevented by strictly adhering to process specifications, especially the heat treatment process, and by implementing reasonable structural design and excellent manufacturing practices.
The intergranular corrosion sensitivity of 6000 series aluminum alloy structures and components is also closely tied to their operating environment. It is essential to give full consideration to the design of structures.








