Have you ever wondered about the vast array of metals that make up our world? In this fascinating blog post, we’ll embark on a journey to explore the different types of metals, from the common to the rare. Our expert mechanical engineer will guide you through the unique properties and applications of each metal, providing insights that will leave you captivated. Get ready to discover a world of metallic wonders!

The following list of metals is categorized using different approaches. As mentioned earlier, there are over 90 distinct types of metals found on our planet.
You can download a PDF version of this list at the bottom of the table.
| Category | Metals |
|---|---|
| Ferrous Metals | Iron, Chromium, Manganese |
| Non-Ferrous Metals | Aluminum, Magnesium, Potassium, Sodium, Calcium, Strontium, Barium, Copper, Lead, Zinc, Tin, Cobalt, Nickel, Antimony, Mercury, Cadmium, Bismuth, Gold, Silver, Platinum, Ruthenium, Rhodium, Palladium, Osmium, Iridium, Beryllium, Lithium, Rubidium, Cesium, Titanium, Zirconium, Hafnium, Vanadium, Niobium, Tantalum, Tungsten, Molybdenum, Gallium, Indium, Thallium, Germanium, Rhenium, Lanthanum, Cerium, Praseodymium, Neodymium, Samarium, Europium, Gadolinium, Terbium, Dysprosium, Holmium, Erbium, Thulium, Ytterbium, Lutetium, Scandium, Silicon, Boron, Selenium, Tellurium, Arsenic, Thorium |
| Common Metals | Iron, Aluminum, Copper, Zinc |
| Rare Metals | Zirconium, Hafnium, Niobium, Tantalum |
| Light Metals | Titanium, Aluminum, Magnesium, Potassium, Sodium, Calcium, Strontium, Barium (Density < 4500kg/m³) |
| Heavy Metals | Copper, Nickel, Cobalt, Lead, Zinc, Tin, Antimony, Bismuth, Cadmium, Mercury (Density > 4500kg/m³) |
| Precious Metals | Gold, Silver, Platinum Group Metals |
| Metalloid Elements | Germanium, Antimony, Polonium |
| Rare Metals | Rare Light Metals (Lithium, Rubidium, Cesium), Rare Refractory Metals (Zirconium, Molybdenum, Tungsten), Rare Dispersed Metals (Gallium, Indium, Germanium, Thallium), Rare Earth Metals (Scandium, Yttrium, Lanthanide Series), Radioactive Metals (Radium, Francium, Polonium, Uranium, Thorium) |
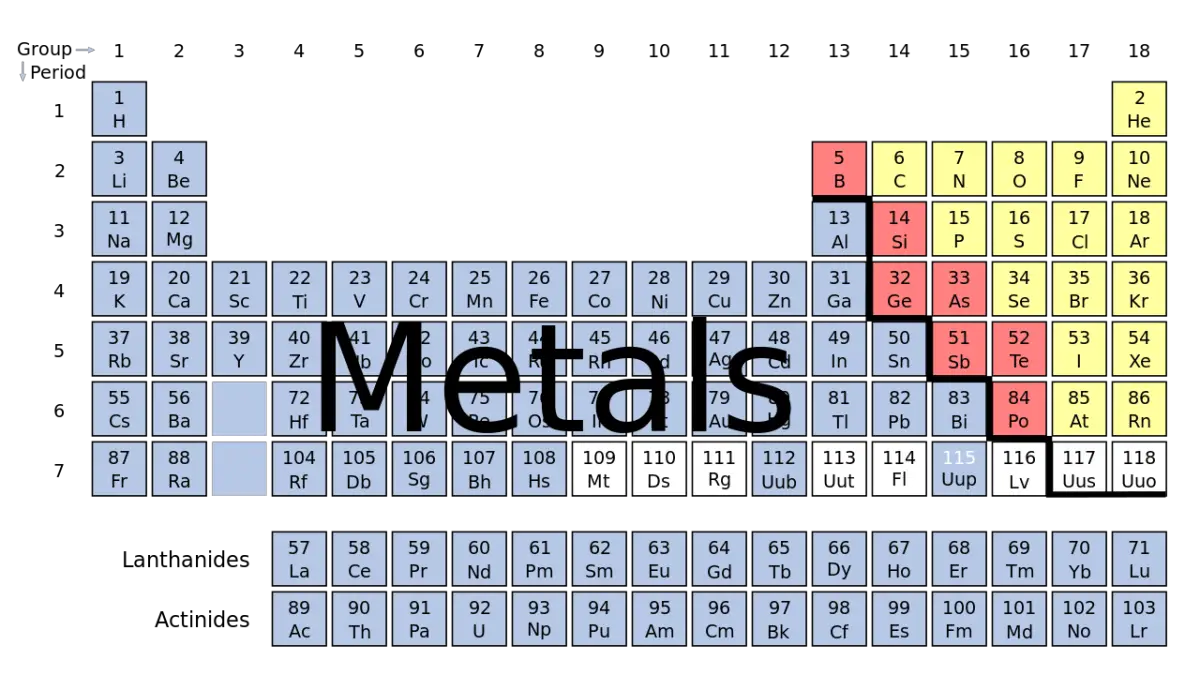
Understanding the total number of existing metals can be quite complex due to the extensive range of elements. As of now, there are a total of 118 known elements in the periodic table, out of which approximately 90 are classified as metals. This includes three elements often referred to as metalloids or semi-metals: boron, silicon, and arsenic.
To simplify the understanding, metals are usually divided into two main categories: ferrous and non-ferrous metals. This classification system is widely accepted in the United States, Britain, and Japan.
Ferrous Metals
Ferrous metals contain iron and are known for their strength and durability. Common examples include:
Non-Ferrous Metals
Non-ferrous metals do not contain iron and are typically more resistant to rust and corrosion. Examples include:
Related reading: Ferrous vs Non-ferrous Metals
In the past, the former Soviet Union and some Eastern European countries categorized metals into two groups based on their color: black metals and colored metals. Similarly, this color-based classification is still used in China. However, this approach lacks scientific validity.
Metals are now categorized based on their properties and applications into four distinct groups:
Heavy Metals:
Heavy metals are defined as metals with a density greater than 4.5 g/cm³. Examples include:
Light Metals:
Light metals have a density less than 4.5 g/cm³. Examples include:
Precious Metals:
Precious metals are highly valued for their low impurity levels, intricate purification processes, and high worth. These metals are considered more valuable than regular metals. Examples include:
Rare Metals:
Rare metals include relatively uncommon elements, such as rare light metals, refractory metals, dispersed metals, and rare earth metals. Examples include:
Radioactive Metals:
It is important to note that there is also a category of radioactive metals that can be harmful to human health. Extended exposure to these metals can result in sickness or even death. Examples include:
Conclusion
This article aims to provide a comprehensive list of different types of metals, covering nearly all the elements found in the periodic table of chemical elements. Moreover, we will present a thorough overview of the features and applications of these metals.
Let’s begin.

Iron is a metallic element with the atomic number 26 and is represented by the chemical symbol Fe, derived from its Latin name ‘Ferrum’. It has an average relative atomic mass of 55.845 atomic mass units (amu). Iron is one of the most abundant elements on Earth and plays a crucial role in various industrial applications, particularly in the production of steel.
Iron is essential in numerous fields due to its versatility and abundance. It is a primary component in the manufacturing of steel, which is an alloy of iron and carbon. Steel is fundamental to construction, automotive, and various other industries due to its strength and durability.

Chromium is a metallic element belonging to Group 6B in the periodic table of elements, with a chemical symbol of Cr and an atomic number of 24. Its name is derived from the Greek word for “color”, which is due to the colorful nature of chromium compounds.
This steel-gray metal is the hardest metal found in nature. Chromium is only present in small amounts in the Earth’s crust, ranking 17th in abundance at only 0.01%. Free, naturally occurring chromium is extremely rare and is primarily found in chromite.

Manganese is a transition metal with a chemical symbol of Mn and atomic number 25. It appears as a grayish-white, hard, brittle, and shiny element.
While pure manganese is slightly softer than iron, it becomes firm and brittle when it contains small amounts of impurities and can easily oxidize in damp environments.
Manganese is widely distributed in nature, with soil typically containing around 0.25% of this element. Certain foods, such as tea, wheat, and hard-shelled fruits, contain higher amounts of manganese.
Aluminum, symbolized by Al, is a ductile, silver-white light metal commonly used to create various products such as rods, sheets, foil, powder, strips, and filaments.
In humid air, aluminum can form an oxide film that protects against corrosion. When heated in air, aluminum powder can ignite, producing a bright white flame. It is soluble in dilute solutions of sulfuric acid, nitric acid, hydrochloric acid, sodium hydroxide, and potassium hydroxide, but insoluble in water.
Aluminum has a relative density of 2.70, and its melting point and boiling point are 660°C and 2327°C, respectively.
Notably, aluminum is the most abundant metal element in the Earth’s crust, ranking third only to oxygen and silicon.

Magnesium is a metallic element represented by the chemical symbol Mg. It was first produced by the British chemist, Sir Humphry Davy, in 1808 by reducing magnesium oxide with potassium.
As an alkaline earth metal, magnesium is a light, silver-white metal that exhibits reactive chemical properties. It reacts with acids to produce hydrogen and has some ductility and heat dissipation capabilities.
Magnesium is naturally abundant and is an essential element for the human body.

Potassium is an alkali metal with the symbol K and atomic number 19. It belongs to Group 1A of the fourth period in the periodic table of elements.
This soft, waxy metal has a silvery-white appearance and can be easily cut with a knife. It has a low melting and boiling point and a lower density than water. Potassium exhibits highly reactive chemical properties, even more so than sodium.
Sodium, also known by its symbol Na and common name Sodium, is a metallic element located in Group 1A of the third period of the periodic table. It is a representative of the alkali metal elements.
Sodium has a soft texture and exhibits highly reactive chemical properties. When it comes into contact with water, it reacts vigorously to produce sodium hydroxide and release hydrogen gas.

Calcium is a metallic element with an atomic number of 20 and the symbol Ca. It is located in Group 2A and the fourth period of the periodic table of elements.
At room temperature, calcium is a silver-white solid with highly reactive chemical properties. Due to its reactivity, it is primarily found in nature as ions or compounds.

Strontium is a chemical element with the symbol Sr, discovered in 1791-1792 by British chemist and physician Sir Henry Hope while studying an ore. He named it strontia (strontium earth) after its place of origin, Strontian.
Strontium is a silver-white, alkaline earth metal with a yellow luster. It is utilized in the production of alloys, photocells, analytical chemical reagents, fireworks, and other applications.
One of its isotopes, strontium-90, is radioactive and has a half-life of 28.1 years, making it useful as a radiation source.

Barium is an alkaline earth metal element with the chemical symbol Ba, located in Group 2A of the sixth period in the periodic table. It is a soft, silver-white metal with a lustrous appearance and is the most reactive element among the alkaline earth metals.
Due to its highly reactive nature, barium is not found in nature in its pure form. Instead, the most common minerals of barium found in nature are barite (barium sulfate) and witherite (barium carbonate), both of which are insoluble in water.
Although barium was recognized as a new element in 1774, it was not classified as a metal element until after the invention of electrolysis in 1808.
Barium compounds are used in fireworks to produce green coloration based on the principle of flame reaction.

Copper is a transition element and a metal with the chemical symbol Cu and atomic number 29.
In its pure form, copper is a soft metal with a red-orange color and a metallic luster when freshly cut. Its elemental form is purplish-red.
Copper is highly ductile and has excellent thermal and electrical conductivity. Due to these properties, it is widely used in electrical and electronic components, as well as cables. It can also be used in construction materials and can form many alloys, including bronze and brass, which have low resistivity and excellent mechanical properties.
Copper is a durable metal that can be recycled multiple times without losing its mechanical properties.
Related reading: Types of Brass

Lead is a metallic chemical element with the symbol Pb, atomic number 82, and atomic weight 207.2. It is the heaviest non-radioactive element, with a face-centered cubic crystal structure. Lead is a heavy, non-ferrous metal that is corrosion-resistant.
Lead has several advantages, including a low melting point, high resistance to corrosion, impenetrability to X-rays and gamma rays, and good plasticity. Because of these properties, it is commonly processed into sheets and pipes and used in various industries, such as chemical manufacturing, cable production, battery fabrication, and radiation protection.

Zinc is a chemical element with the symbol Zn and atomic number 30. It belongs to Group 12 of the fourth period in the periodic table of elements. Zinc is a light gray transition metal and is the fourth most commonly used metal in modern industry. It is an essential metal in the production of batteries.

The metallic element known as stannum is commonly referred to as tin in English, and its elemental symbol is Sn.
Tin is an inorganic substance that typically appears as a low-melting-point metal with a silver-white luster, in its most common form, white tin.
In compounds, tin may exhibit a valency of either two or four, and it is not easily oxidized in air at room temperature.
The natural occurrence of tin is primarily in the form of dioxide (cassiterite) and various sulfides, such as stannic sulfide.
Related reading: Types of tin

Cobalt, symbolized by Co, is a ferromagnetic metal with a silvery-white surface and a slightly pink hue. It is located in the eighth group and fourth period of the periodic table, with an atomic number of 27 and an atomic weight of 58.9332.
Cobalt has a close-packed hexagonal crystal structure and can commonly have a valence of +2 and +3. It is a shiny, steel-gray metal that is relatively hard and brittle, ferromagnetic, and loses its magnetism when heated to 1150 ℃. At room temperature, it is inert to water and stable in humid air.
When heated to temperatures above 300 ℃ in air, cobalt oxide (CoO) undergoes oxidation and is converted to cobalt oxide (Co3O4) with a bright white heat. Fine cobalt metal powder, produced by hydrogen reduction, can spontaneously combust into cobalt oxide in air.
Cobalt is a crucial raw material used in the manufacturing of heat-resistant alloys, hard alloys, anti-corrosion alloys, magnetic alloys, and various cobalt salts.

Nickel is a hard, ductile, and ferromagnetic metal that possesses a high shine and excellent resistance to corrosion. It is a ferrophilic element and is abundant in the Earth’s core, which primarily comprises iron and nickel. The nickel content in iron-magnesian rocks in the crust is higher than that of aluminosilicate rocks. For instance, peridotite has a nickel content that is 1000 times higher than that of granite, while gabbro has 80 times the nickel content of granite.

Antimony is a metallic element with the chemical symbol Sb and atomic number 51. It is a silvery-white, shiny, hard, and brittle metal that can be formed into rods, blocks, powder, and other shapes. Antimony has a scaly crystal structure and loses its luster over time when exposed to humid air. Upon exposure to high temperatures, it burns into white antimony oxide. Antimony is soluble in aqua regia and concentrated sulfuric acid. It has a relative density of 6.68, a melting point of 630°C, and a boiling point of 1635°C. In addition, its atomic radius is 1.28 angstroms, and its electronegativity is 2.2.

Mercury, denoted by the chemical symbol Hg, is the 80th element in the periodic table and belongs to Group 12 and the 6th period.
What makes mercury unique is that it is the only metal that exists in liquid form at normal temperature and pressure. However, gallium (symbol Ga, element 31) and cesium (symbol Cs, element 55) also exist as liquids at room temperature (29.76°C and 28.44°C, respectively).
Mercury has a shiny, silvery-white appearance and is a dense liquid with stable chemical properties. It is insoluble in both acids and bases.
At room temperature, mercury can evaporate, and both mercury vapor and its compounds are highly toxic, leading to chronic health effects.
Mercury has a long history of use and is still widely used today.

Cadmium is a heavy, non-ferrous metallic element with the chemical symbol Cd and atomic number 48. It is a silver-white metal that possesses excellent neutron-absorbing properties. Cadmium rods are useful for slowing down the chain fission reaction rate in nuclear reactors. Additionally, it is utilized in zinc-cadmium batteries.
The sulfide form of cadmium is bright in color and is employed to produce the yellow pigment known as cadmium yellow.

Bismuth is a metallic element represented by the chemical symbol Bi and atomic number 83. It belongs to Group VA in the 6th period of the periodic table.
Bismuth has a unique appearance with its silver-white to pink color, and it is a brittle metal that can be easily crushed. It exhibits relatively stable chemical properties.
Bismuth can be found in its free metallic form as well as in various minerals in nature.

Gold (symbol: Au, atomic number: 79) is a metallic element often referred to as a precious metal due to its historical use as a form of currency, a means of preserving value, and as jewelry.
Naturally occurring gold can be found in the form of nuggets or grains within rocks, underground veins, and alluvium.
As one of the monetary metals, gold is solid at room temperature and is known for its high density, softness, brightness, and resistance to corrosion. It is the second most ductile metal, after platinum.

Silver, denoted by its chemical symbol Ag, is a transition metal that has been utilized since ancient times and is recognized as a significant precious metal.
Although silver can be found naturally, it is mainly available in silver ore in a chemical form. It possesses fairly stable physical and chemical characteristics, including excellent thermal and electrical conductivity. This soft and malleable metal reflects over 99% of light, making it highly reflective. Due to its numerous crucial applications, silver retains its value as a precious metal.

Platinum is a chemical element represented by the symbol Pt and is considered one of the precious metals. Belonging to the platinum series of elements, it is commonly referred to as simply “platinum.” With an atomic weight of 195.078 and an atomic number of 78, it is a transition metal.
Platinum has a melting point of 1772°C, a boiling point of 3827°C, and a density of 21.45 g/cm³ at 20°C. It is relatively soft and possesses good ductility, thermal conductivity, and electrical conductivity.
Sponge platinum is a gray, sponge-like material with a large specific surface area and a strong absorption capacity for gases, especially hydrogen, oxygen, and carbon monoxide. Powdered platinum black can absorb a significant amount of hydrogen.
Ruthenium is a rare, multivalent metal element that is recognized for its hard, brittle, and light gray appearance. It has the chemical symbol Ru and belongs to the platinum group metals.
Despite being present in the earth’s crust, ruthenium is one of the rarest metals, with a concentration of only one billionth. It is renowned for its stable properties and high resistance to corrosion.
Ruthenium has the ability to withstand corrosion from hydrochloric acid, sulfuric acid, nitric acid, and aqua regia at room temperature.
Although ruthenium is the least expensive of the platinum group metals, it is still less abundant than other metals like platinum and palladium.

Rhodium is a hard, silvery-white metal represented by the chemical symbol Rh. It belongs to the platinum group elements and is known for its high reflectivity.
Normally, Rhodium metal does not form oxides, but when it is in a molten state, it can absorb oxygen and release it upon solidification.
Compared to platinum, rhodium has a higher melting point and a lower density. Moreover, it is insoluble in most acids and completely insoluble in nitric acid. It is only slightly soluble in aqua regia.
Palladium is a transition metal belonging to the platinum group, with the chemical symbol Pd. It is located in Group VIII of the fifth period of the periodic table.
In its pure form, palladium is a silver-white metal that has a soft texture and good ductility and plasticity. This makes it easy to forge, roll, and draw into different shapes.
Palladium has the unique characteristic of being able to absorb hydrogen gas, which results in a significant increase in volume. However, this property can also cause the metal to become brittle and even break into fragments.

Osmium is a chemical element with the symbol Os and atomic number 76. It belongs to Group VIII in the 6th period of the periodic table and has a relative atomic mass of 190.23.
As a member of the platinum group, osmium is a heavy metal with the highest density of all elements.

Iridium is a metallic element with the chemical symbol Ir and atomic number 77. Its atomic weight is 192.22, and its name is derived from the Latin word for “Rainbow.”
In the Earth’s crust, iridium is scarce, occurring at a concentration of only 1/10 million. It is typically dispersed in various ores and can be found in alluvial and sandy deposits, along with other elements in the platinum series.
Beryllium is a chemical element represented by the symbol Be and has an atomic number of 4. It is classified under the second main group and second period of the periodic table.
This grayish-white alkaline earth metal belongs to the hexagonal system and is known for its hardness and low coefficient of thermal expansion. However, it should be handled with care since beryllium and its compounds are highly toxic.
Beryllium is an amphoteric metal and can dissolve in both acids and bases. Its applications are diverse, ranging from its use as a material in atomic energy reactors and aerospace engineering to its incorporation in various alloys and as a component in X-ray transmission windows.

Lithium is a metallic element with the chemical symbol Li and a soft, silver-white appearance. It has the lowest density of all metals.
Lithium is used in various applications, including atomic reactors, light alloys, and batteries. Unlike other alkali metals, lithium and its compounds have atypical properties due to their high charge density and stable helium-type double electron layer. Consequently, they are easily polarized by other molecules or ions but difficult to polarize themselves.
This unique characteristic affects the stability of lithium and its compounds. Lithium has the most negative electrode potential among all known elements, including radioactive ones, which makes it the most reactive metal.

Rubidium is a light, silvery-white metal denoted by the chemical symbol Rb. It has a soft and waxy texture and displays more active chemical properties than potassium.
When exposed to light, rubidium is known to emit electrons. It reacts vigorously with water, producing rubidium hydroxide and hydrogen. Additionally, it readily reacts with oxygen to form complex oxides.
Due to the significant heat generated during the reaction with water, there is a risk of hydrogen igniting immediately. As a precaution, pure rubidium metal is typically stored in sealed glass containers to prevent contact with air or moisture.

Cesium is an element in the periodic table with the symbol Cs and atomic number 55. It is classified as a Group IA element in the 6th period.
Cesium in its elemental form is a light golden-yellow, reactive metal with a low melting point. It is highly sensitive to air and prone to oxidation.
When cesium comes into contact with water, it reacts violently and may explode, producing hydrogen. In nature, cesium only exists as a salt and is rarely found on land and sea.
Cesium is an important material in the production of vacuum devices and photocells. The radioactive isotope Cs-137 was among the pollutants leaked from the Fukushima Daiichi nuclear power plant in Japan.
Cesium is the most metallic of all known elements, including radioactive ones. It’s worth noting that lithium is the most reactive element.

Titanium is a chemical element with the symbol Ti and atomic number 22. It belongs to Group IVB in the 4th period of the periodic table and is a silvery-white transition metal known for its lightweight, high strength, metallic luster, and resistance to corrosion by moist chlorine.
However, exposure to dry chlorine can cause a violent chemical reaction in titanium, even at temperatures below 0°C. This reaction produces titanium tetrachloride and decomposes to produce titanium dichloride, which can ignite in extreme cases. Therefore, titanium can only remain stable when the water content in chlorine is higher than 0.5%.
Titanium is considered a rare metal due to its dispersed occurrence in nature and the difficulty in extraction. Despite this, it is relatively abundant and ranks 10th among all elements. The main titanium ores, ilmenite and rutile, are widely found in the crust and lithosphere. Additionally, titanium can be found in almost all living organisms, rocks, water, and soil.

Zirconium is a chemical element with the symbol Zr and atomic number 40. It is a light gray metal with a high melting point.
When exposed to air, zirconium’s surface quickly develops an oxide film that gives it a lustrous appearance, similar to steel. It also has outstanding resistance to corrosion and is soluble in both hydrofluoric acid and aqua regia.
Zirconium can react with both non-metallic and metallic elements at high temperatures, forming solid solutions.

Vanadium is a metallic element with the symbol V. It is a silver-gray metal that belongs to Group 5 in the periodic table of elements. Vanadium has an atomic number of 23 and an atomic weight of 50.9414. It has a body-centered cubic crystal structure and exhibits common valences of +5, +4, +3, and +2.
Vanadium is classified as a refractory metal due to its high melting point. Additionally, it is ductile, hard, and non-magnetic. Vanadium is also highly resistant to hydrochloric acid and sulfuric acid, and it exhibits better resistance to corrosion from gases, salts, and water compared to most types of stainless steel.

Niobium is a transition metal element with the chemical symbol Nb and atomic number 41. It is a shiny, gray metal.
In its pure form, niobium is highly ductile. However, its hardness increases as the impurity content increases. Additionally, niobium has a very low cross-section for capturing thermal neutrons, which makes it highly valuable in the nuclear industry.

Tantalum is a metallic element with an atomic number of 73 and a chemical symbol of Ta. Its elemental form is a steel-gray metal that exhibits high resistance to corrosion.
Tantalum shows no reactivity towards hydrochloric acid, concentrated nitric acid, or aqua regia under both cold and hot conditions. It is primarily found in tantalite, often coexisting with niobium.
Tantalum exhibits moderate hardness and ductility and can be drawn into thin wire or foil. It has a small coefficient of thermal expansion, excellent chemical properties, and high corrosion resistance.
Tantalum is used in the production of evaporation vessels and as an electrode, rectifier, and electrolytic capacitor in electron tubes. In medicine, it is used to create thin sheets or threads for repairing damaged tissue.
The strong corrosion resistance of tantalum is due to the formation of a stable protective film of tantalum pentoxide (Ta2O5) on its surface.

Tungsten is a metallic element with the chemical symbol W and atomic number 74. It belongs to the VIB group of the sixth period in the periodic table of elements.
In nature, tungsten primarily exists in the form of hexavalent cations, which have an ion radius of 0.68 x 10^-10 meters. Due to its small ion radius, high electronegativity, and strong polarization ability, it readily forms complex anions. Thus, tungsten often occurs in the form of complex anions, such as [WO4]^2-, in wolframite or scheelite precipitates.
Tungsten in its elemental form appears as a shiny, silver-white metal with high hardness and a high melting point. It is resistant to corrosion by air at room temperature and possesses relatively stable chemical properties. Tungsten has numerous applications, including the manufacturing of filaments, high-speed cutting alloys, superhard molds, optical and chemical instruments. China holds the world’s largest reserves of tungsten.

Molybdenum is a chemical element with the symbol Mo and atomic number 42. It belongs to the transition metals group and is a crucial trace element for human health, found in various tissues of the body such as the liver and kidneys.
The human body contains about 9mg of molybdenum in total. This silver-white metal is known for its toughness and hardness, and is important for the growth and wellbeing of both plants and animals.

Gallium is a metallic element that exhibits a grayish-blue or silver-white color, represented by the chemical symbol Ga and an atomic weight of 69.723.
Despite having a low melting point, gallium has a high boiling point. When gallium is in its pure liquid form, it has a tendency to supercool and is easily oxidized in air, resulting in the development of an oxide film.

Indium is a metallic element with the symbol “In” and an atomic number of 49. It belongs to Group IIIA in the fifth period of the periodic table.
In its pure state, indium appears as a silvery-white metal with a light blue hue. It is extremely soft and can be easily scratched with a fingernail. Moreover, indium exhibits remarkable malleability and ductility, enabling it to be molded into various forms.
Indium is mainly used as a base material in the manufacture of low melting point alloys, bearing alloys, semiconductors, and electric light sources.

Thallium, symbolized by Tl and atomic number 81, is a Group IIIA element that belongs to the sixth period of the periodic table.
Being a rare element, it is found in small quantities in the natural environment. Thallium slowly dissolves in hydrochloric acid and dilute sulfuric acid, but it rapidly dissolves in nitric acid.
The primary compounds of thallium include oxides, sulfides, halides, and sulfates. Thallium salts are colorless and tasteless crystals that dissolve in water, forming thallium compounds.
Thallium is relatively more stable in water or paraffin compared to air.

Germanium is a chemical element with the symbol Ge, atomic number 32, and atomic weight 72.64. It is located in the 4th period and Group IVA of the periodic table of elements.
Germanium is a shiny, hard, grayish-white metalloid. It belongs to the carbon group, and its chemical properties are similar to those of tin and silicon, which are also in the same group.
Germanium is insoluble in water, hydrochloric acid, and dilute caustic solutions, but it is soluble in aqua regia, concentrated nitric acid, or sulfuric acid. It has amphoteric properties and is soluble in molten alkali, peroxide alkali, alkali metal nitrate, or carbonate. It is relatively stable in air.
There are five stable isotopes of germanium found in nature: 70Ge, 72Ge, 73Ge, 74Ge, and 76Ge. When germanium reacts with oxygen above 700°C, it forms GeO2. When it reacts with hydrogen above 1000°C, it can ignite in chlorine or bromine.
Germanium is an excellent semiconductor and can be used for detecting high-frequency currents and rectifying AC electricity. It can also be used as an infrared optical material, in precision instruments, and as a catalyst. Germanium compounds can be used to make fluorescent plates and various glasses with a high refractive index.

Rhenium is a chemical element denoted by the symbol Re and has an atomic number of 75. It is a dense, silvery-white metal belonging to the sixth period of transition metals in the periodic table of elements.
Rhenium is an incredibly rare element that is found in the Earth’s crust with an estimated average concentration of just one billionth. It is also known for having one of the highest melting and boiling points of all the elements.
The process of refining molybdenum and copper produces rhenium as a byproduct. Rhenium has chemical properties that are comparable to manganese and technetium.
Compounds of rhenium have oxidation states that range from -3 to +7, with -3 being the lowest and +7 being the highest.

Lanthanum is a rare earth metallic element with the chemical symbol La, an atomic number of 57, and an atomic weight of 138.90547. The name of the element comes from the Greek language and originally means “to lie hidden.”
Lanthanum has a silver-gray luster and a soft texture, with a density of 6.162 g/cm3. Its melting point is 920°C, and its boiling point is 3464°C at atmospheric pressure. It exhibits active chemical properties and loses its metallic luster quickly when exposed to air, forming a layer of blue oxide film. However, this film is unable to protect the metal, leading to continued oxidation and the formation of white oxide powder.
Lanthanum reacts slowly with cold water, is soluble in acid, and can react with various nonmetals. Typically, the metal is stored in mineral oil or a rare gas.
The Earth’s crust contains 0.00183% of lanthanum, making it the second most abundant rare earth element after cerium. There are two natural isotopes of lanthanum: lanthanum-139 and radioactive lanthanum-138.

Cerium is a rare earth element with an atomic number of 58. It belongs to the IIIB lanthanide group in the sixth period of the periodic table and is represented by the chemical symbol Ce. In its elemental form, it appears as a silver-gray, reactive metal.
It is worth noting that cerium is prone to spontaneous combustion when in powder form and can dissolve in acids and reducing agents.

Praseodymium is a rare earth metal with an atomic number of 59. Its name originates from the Greek language, meaning “green.” Praseodymium has a hexagonal crystal structure.
Compared to lanthanum, cerium, neodymium, and europium, praseodymium exhibits greater corrosion resistance in air. However, when exposed to air, it still forms a layer of fragile green oxide. Pure praseodymium should be stored in mineral oil or a sealed plastic container.
Praseodymium is utilized in petroleum catalytic cracking. The addition of praseodymium and neodymium enrichment to a Y-zeolite molecular sieve can enhance the activity, selectivity, and stability of a petroleum cracking catalyst.
Similar to other rare earth elements, praseodymium has low toxicity and is not essential for biological processes.

Neodymium, symbolized as Nd and with an atomic number of 60, belongs to the lanthanide series of elements. It is a silver-white metal and one of the most reactive rare earth metals.
Neodymium has a density of 7.004 g/cm³ and a melting point of 1024°C. It is also paramagnetic and quickly darkens when exposed to air, forming oxides. It reacts slowly with cold water and rapidly with hot water.
Neodymium-doped yttrium aluminum garnet and neodymium glass can replace ruby as laser materials, while neodymium and praseodymium glass can serve as goggles.
Neodymium is a crucial element in the rare earth industry and plays a significant role in regulating the rare earth market.

Samarium is a metallic element with the chemical symbol Sm and an atomic number of 62. It has a silver-white color, medium hardness, and is prone to oxidation when exposed to air.
As a representative of the lanthanide series, samarium typically exists in an oxidation state of +3. The most prevalent samarium compounds include SmO, SmS, SmI2, and SmTe.
Samarium is not known to have any significant biological effects and exhibits only slight toxicity.

Europium is a metallic element with a silvery-white color that can be oxidized into an almost white oxide. It has a melting point of 822°C, a boiling point of 1597°C, and a density of 5.2434 g/cm³.
Among the rare earth elements, europium is the softest and most volatile, and it is also the most reactive metal. When exposed to air at room temperature, it loses its metallic luster and quickly oxidizes into a powder.
Europium reacts violently with cold water, producing hydrogen. Additionally, it can react with boron, carbon, sulfur, phosphorus, hydrogen, and nitrogen.
Europium has many practical applications. It is widely used in the production of reactor control and neutron protection materials, as well as in the atomic energy industry as a phosphor for color TV and in the production of europium (Eu) laser materials.
Europium is one of the rarest rare earth elements on Earth, with a content of only 1.1 ppm. It is a soft, shiny, steel-gray metal with strong ductility and malleability, making it easily processed into various shapes. It resembles lead in appearance and feel but is slightly heavier.

Gadolinium is a metallic element represented by the symbol Gd, with an atomic number of 64 and an atomic weight of 157.25. It has a silvery-white appearance and is ductile in nature. The element is named after the Finnish scientist Gadolin, who made significant contributions to the study of lanthanides.
Gadolinium was first isolated in Malaya, Switzerland, in 1880, and its pure form was prepared and named by the French chemist Bouvabodrand in 1886. It is primarily found in minerals such as monazite and bastnaesite, and its abundance in the earth’s crust is only 0.000636%.
Gadolinium has a wide range of applications in fields such as medicine, industry, and nuclear technology, among others.

Terbium is a member of the lanthanide series and is represented by the chemical symbol Tb with an atomic number of 65. It is situated in Group III of the periodic table’s sixth period and has a silver-white metallic appearance in its elemental form.
Being a rare earth metal, terbium is toxic and has only one stable isotope that occurs naturally, alongside 20 additional radioisotopes. It possesses a hexagonal crystal structure and dissolves in dilute acid, but reacts slowly with water.
Due to its high reactivity, terbium must be stored in a container filled with an inert gas or a vacuum container.
Dysprosium is a soft, silvery-white metal represented by the chemical symbol Dy. It has a melting point of 1412°C, a boiling point of 2562°C, and a density of 8.55g/cm³. It is even capable of exhibiting superconductivity near absolute zero.
Although Dysprosium is relatively stable when exposed to air, it can be readily oxidized by air and water at high temperatures, leading to the formation of dysprosium oxide.
Dysprosium finds widespread applications in various fields, including the manufacturing of new lighting sources like dysprosium lamps, as a control material in reactors, and as a catalyst in the oil refining industry in the form of dysprosium compounds.

Holmium is a metallic element with the chemical symbol Ho, atomic number 67, and atomic weight 164.93. It was named after its discoverer’s birthplace, Stockholm, who first identified it from the spectrum of erbium earth in 1878. The following year, Clive of Sweden separated holmium from erbium earth using chemical methods.
The concentration of holmium in the Earth’s crust is 0.000115%, and it is found in monazite and rare earth ores, along with other rare earth elements. Holmium-165 is the only stable isotope of holmium. It is a silver-white metal that is soft and ductile, with a melting point of 1474°C, a boiling point of 2695°C, and a density of 8.7947 g/cm³.
Although holmium is stable in dry air, it oxidizes quickly at high temperatures. Holmium oxide is the most paramagnetic substance known, and holmium compounds can be used as additives for new ferromagnetic materials. Holmium iodide is used to make metal halide lamps, such as holmium lamps, and holmium lasers are widely used in the medical field.

Erbium is an element on the periodic table with the symbol Er and atomic number 68. It belongs to the lanthanide series and is located in Group III B of the 6th period, with an atomic weight of 167.26. The element’s name comes from the location of its discovery, yttrium earth.
Erbium oxide was first discovered in yttrium earth by Swedish scientist Mossander in 1843 and was officially named in 1860. The content of erbium in the earth’s crust is 0.000247%, and it is present in many rare earth minerals. There are six natural isotopes of erbium, which are 162, 164, 166, 167, 168, and 170.

Thulium is a soft, silvery-white metal with the chemical symbol TM. It is malleable and can be easily cut with a knife. Thulium has a melting point of 1545°C, a boiling point of 1947°C, and a density of 9.3208.
It is relatively stable in air, and its oxide form appears as a light green crystal. Thulium has an atomic number of 69 and an atomic weight of 168.93421. The element’s name is derived from the country where it was discovered.
Thulium is the least abundant of the rare earth elements, with a concentration in the Earth’s crust of only 2 parts per 100,000. It is primarily found in yttrium-phosphorus ores and black rare earth mines. The only stable natural isotope of thulium is 169.
Thulium has various applications in different fields, including high-intensity power generation light sources, lasers, high-temperature superconductors, and others.

Ytterbium is a metallic element with the chemical symbol Yb, atomic number 70, and an atomic weight of 173.04. Its name is derived from the place where it was discovered.
The concentration of ytterbium in the Earth’s crust is 0.000266%. It is primarily found in yttrium-phosphorus ores and black rare earth mines. There are seven natural isotopes of ytterbium.
Lutetium is a metallic element with the chemical symbol Lu. It is a silver-white metal that is the hardest and densest of all the rare earth elements, with a melting point of 1663°C, a boiling point of 3395°C, and a density of 9.8404. Lutetium is relatively stable in air, and its oxide form is a colorless crystal that dissolves in acid to form a corresponding colorless salt.
Although Lutetium has limited natural reserves, it has several uses, mainly for research purposes. It is soluble in dilute acids and reacts slowly with water. Its salts are colorless, and its oxides are white. The two naturally occurring isotopes of Lutetium are 175Lu, with a half-life of 2.1 x 1010 years, and 176Lu.
Because of its limited natural reserves, Lutetium is an expensive element.

Scandium is a chemical element with the symbol Sc and atomic number 21. It is a soft, silvery-white transition metal that is sometimes alloyed with gadolinium and erbium.
The production of Scandium is highly limited, and its concentration in the Earth’s crust is about 0.0005%. It is commonly utilized in the production of specialized glasses and lightweight, heat-resistant alloys.

Yttrium is a grayish-black metal identified by the chemical symbol Y. It is considered the first rare earth metal element discovered and is known for its ductility. Yttrium readily reacts with hot water and can be dissolved in dilute acids. Additionally, it is used in the production of special glasses and alloys.

Thorium is a radioactive metallic element identified by the chemical symbol Th. Its potential as a nuclear fuel lies in its ability to transform into uranium-233 when subjected to neutron bombardment. Thorium has a soft texture and gray luster and is characterized by its active chemical properties. It is widely distributed throughout the Earth’s crust and is considered a promising energy material for its potential applications in the field of nuclear energy.

Hafnium is a metallic element with the chemical symbol Hf, atomic number 72, and atomic weight 178.49. It appears as a shiny, silver-gray transition metal in its pure form. There are six stable isotopes of hafnium found in nature: hafnium-174, hafnium-176, hafnium-177, hafnium-178, hafnium-179, and hafnium-180.
Hafnium is relatively unreactive and does not react with dilute hydrochloric acid, dilute sulfuric acid, or strong alkali solutions. However, it is soluble in hydrofluoric acid and aqua regia. The concentration of hafnium in the Earth’s crust is relatively low, at only 0.00045%. It is often found in association with zirconium in nature.

Silicon, also known by its former name, silicium, is a chemical element represented by the symbol Si. With an atomic number of 14 and a relative atomic mass of 28.0855, it exists in two forms: amorphous and crystalline silicon.
In the periodic table, silicon is situated in the third period and is categorized as a metalloid element in group IVA. It is a highly abundant element, ranking eighth in the universe.
Despite its abundance, pure silicon is rare in nature. It is commonly found in complex silicates or silica present in rocks, gravel, and dust.
Silicon is the second most abundant element in the Earth’s crust, accounting for 26.4% of the total mass. Oxygen is the most abundant element, comprising 49.4% of the crust.

Selenium is a non-metallic element represented by the chemical symbol Se. It is located in group VIa of the fourth period in the periodic table of elements (element 34). Selenium has numerous applications, including acting as a photosensitive material, a catalyst in the electrolytic manganese industry, and an essential nutrient for animals, as well as a beneficial nutrient for plants.
In nature, selenium exists in two forms: inorganic selenium and plant-active selenium. Inorganic selenium is composed of sodium selenite and sodium selenate, which are obtained from metal deposit by-products.
Plant-active selenium, on the other hand, results from the combination of selenium and amino acids through biotransformation. It is often present in the form of selenomethionine.

Selenium is a non-metallic element represented by the chemical symbol Se. It belongs to group VIa of the periodic table of elements and is located in the fourth period as element 34. Selenium has multiple applications, including its use as a photosensitive material, a catalyst in the electrolytic manganese industry, and as a vital nutrient for animals and a beneficial nutrient for plants.
In nature, selenium occurs in two forms: inorganic selenium and plant-active selenium. Inorganic selenium is obtained as a by-product from metal deposits and includes sodium selenite and sodium selenate.
On the other hand, plant-active selenium is produced through biotransformation by combining selenium with amino acids. It is typically found in the form of selenomethionine.

Arsenic, also known as As, is a non-metallic element found in Group VA of the fourth period in the periodic table of elements. It has an atomic number of 33 and exists in three different allotropic forms: gray, black, and yellow arsenic.
This element is widespread in nature, and many arsenic-containing minerals have been discovered. Arsenic and its compounds are used for various purposes, including in pesticides, herbicides, insecticides, and alloys. However, its compound arsenic trioxide is highly toxic.

Boron, represented by the symbol B, is a chemical element present in the Earth’s crust with a concentration of only 0.001%. It is commonly found in the form of black or silver-gray solids with a black crystalline structure, and has a hardness second only to diamonds, but is brittle in texture.
What sets boron apart from other elements is its unusually high coordination number in its hydride, which is a result of its electron deficiency. As a result, it has the most complex elemental hydrides.

Radium, symbolized as Ra, is a highly radioactive element that belongs to the 7th period, group IIA, and has an atomic number of 88 on the periodic table of elements.
Although pure radium metal is almost colorless, it reacts with nitrogen in the air to form black radium nitride (Ra3N2).
All isotopes of radium exhibit strong radioactivity, with radium-226 being the most stable isotope. It has a half-life of approximately 1600 years and decays into radon-222.
The decay of radium produces ionizing radiation that causes fluorescent substances to glow.
Madame Curie is credited with the discovery of radium, which has made significant contributions to science.
Francium is a radioactive element denoted by the chemical symbol Fr and has an atomic number of 87. It is formed via the alpha decay of actinium-227 and can be found in small quantities in nature.
All 21 isotopes of francium that are currently known are radioactive and exhibit very short half-lives. Among these, francium-223 has the longest half-life of 21 minutes and emits beta particles. The other three isotopes with relatively longer half-lives are francium-212, francium-222, and francium-221, having half-lives of 19.3, 14.8, and 4.8 minutes, respectively.
Polonium is one of the rarest elements known to humans, with a chemical symbol of Po and an atomic number of 84. Its concentration in the Earth’s crust is approximately one hundred trillionth and it is mainly obtained through artificial synthesis.
The silvery-white metal of polonium emits a glow in the dark. It was discovered in 1898 by the renowned scientists Madame Curie and her husband Pierre Curie, and was named after Madame Curie’s homeland of Poland.
Polonium is also known to be one of the most toxic substances in the world.

Uranium is an element with an atomic number of 92 and symbol U. It is the heaviest naturally occurring element.
There are three isotopes of uranium found in nature, all of which are radioactive and have very long half-lives, ranging from hundreds of thousands of years to 4.5 billion years.

Plutonium is a radioactive element with atomic number 94 and symbol Pu. It is a crucial raw material for the atomic energy industry and has various applications, including its use as nuclear fuel and as a fissile agent in nuclear weapons. The core of the atomic bomb dropped on Nagasaki was made of plutonium. Plutonium was first synthesized at the United States National Laboratory in December 1940.
Ferrous metals are metals that contain iron as their main constituent, while non-ferrous metals are metals that do not contain iron as their main constituent. This difference in composition gives these two types of metals different properties and characteristics.
The most commonly used metal in the world is iron. Iron is used extensively in construction, transportation, and manufacturing due to its strength, durability, and low cost. Other commonly used metals include aluminum, copper, and steel.
The strongest metal known is currently tungsten, also known as wolfram, with a tensile strength of up to 1,510 megapascals (MPa). Tungsten has the highest melting point of any metal, as well as excellent corrosion resistance, making it highly valued in a variety of industries, including aerospace, defense, and electronics. However, there are other materials with higher tensile strengths than tungsten, such as carbon nanotubes and graphene, but they are not metals.
The most expensive metal in the world is currently rhodium. As of March 2023, rhodium is trading at around $20,000 per troy ounce, making it more than 10 times more expensive than gold. Rhodium is a rare, silvery-white metal that is primarily used in catalytic converters in automobiles and other industrial applications, as well as in jewelry and other decorative items. Other expensive metals include platinum, gold, and palladium.
Magnetic metals comprise iron, nickel, cobalt, steel, stainless steel, and rare earth metals. Certain materials among them demonstrate permanent magnetism, whereas others, such as stainless steel, only exhibit magnetism if they possess a particular chemical composition.
Iron
Iron is the strongest ferromagnetic metal and is responsible for endowing the Earth with its magnetic field. It is a crucial component of the planet’s core.
Nickel
Nickel is also a common magnetic metal with ferromagnetic properties. Nickel has always been used to make coins.
Cobalt
Cobalt is a ferromagnetic metal that has been widely used over the past century due to its exceptional magnetic properties. It is suitable for producing both soft and hard magnets.
Steel
Steel is ferromagnetic due to its iron content, making it frequently attracted to magnets. Additionally, steel is capable of producing permanent magnets.
Stainless steel
Stainless steel is an alloy steel made by adding chromium to the mix. While some types of stainless steel exhibit magnetic properties, others do not. The magnetic properties of ferritic and martensitic stainless steels are influenced by their composition and molecular structure.
The nickel content is the primary factor that accounts for the variations in magnetic properties among different types of stainless steel.
Rare earth metals
Stainless steel is an alloy steel created by adding chromium to the base metal. However, not all types of stainless steel are magnetic, and the magnetic properties of ferritic and martensitic stainless steel are a result of their chemical composition and molecular structure.
The amount of nickel present in stainless steel is the primary factor that causes variations in its magnetic properties across different types of stainless steel.
Only a limited number of metals on the periodic table exhibit magnetic properties. In contrast, the majority of commonly used metals, such as aluminum, gold, silver, and copper, are non-magnetic.
Aluminium
The crystal structure of aluminum is similar to that of lithium and magnesium, which causes it to be non-magnetic. All three materials are classified as paramagnetic metals.
Gold
Similar to other metals, gold is diamagnetic, meaning it has a slight magnetic attraction to strong magnets. This characteristic is common among all diamagnetic metals, including gold.
Silver
Silver is another non-magnetic metal. The diamagnetism of silver makes it nonmagnetic.
Copper
Copper is not inherently magnetic, but it can interact with magnets in several ways, including the creation of eddy currents. Power plants take advantage of this property of copper to generate electricity.
Heavy metals include mercury, lead, cadmium, gold, silver, copper and iron.
Heavy metals are metals that have a density greater than 4.5 g/cm3. They are highly resistant to biodegradation and can accumulate in the food chain, leading to a hundredfold enrichment in the environment.
When heavy metals enter the human body through food, they can disrupt normal physiological functions and pose a threat to human health. These types of heavy metals are referred to as toxic heavy metals.
In terms of environmental pollution, heavy metals mainly refer to heavy elements with significant biological toxicity such as mercury, cadmium, lead, chromium and metalloid arsenic.
Heavy metals have the potential to strongly interact with proteins and enzymes within the human body, rendering them inactive. Furthermore, they can accumulate in specific organs, resulting in chronic poisoning.
The most precious metal in the world is plutonium, which costs US $113400 per ounce.
Plutonium is a radioactive metal used in the production of fuel pellets for nuclear power plants, as well as an ingredient in creating nuclear weapons.
Why is plutonium so expensive?
Plutonium is a rare element that is typically found in small quantities within uranium ore in nature. However, the majority of plutonium is produced as a by-product of the nuclear power industry, by irradiating uranium in reactors. It is estimated that around 20 tons of plutonium is generated annually through this process.
Due to its limited applications, which include nuclear energy, weapons, and scientific research, and the potential hazards it poses to humans if mishandled, plutonium is subject to strict regulations and is challenging to obtain. Additionally, its acquisition can be quite costly.
Aluminum is the metal element with the highest concentration in the Earth’s crust, accounting for 7.73% of the total. Calcium is the metal element with the highest concentration in the human body, making up 1.5% of its composition. Iron is currently the metal with the highest annual production globally.
Hydrogen is the metal with the lowest density and became the lightest metal after scientists at Edinburgh University produced metallic hydrogen for the first time in January 2016. Osmium has the highest density of all metals, with a density of 22.48 × 10³ kg/m³.
Chromium is the hardest metal with a Mohs hardness of about 9, while cesium is the softest metal with a Mohs hardness of about 0.5. Silver is the most conductive metal.
Titanium is considered the most important metal for the manufacturing of high-speed aircraft and is referred to as “the metal of the 21st century” or “the steel of the future” by scientists. Uranium is the largest radioactive element found in seawater, with the total reserves of terrestrial uranium mines estimated at 2 million tons and the total amount of uranium in the ocean estimated at 4 million tons.
Tin has the most isotopes, with 10 stable isotopes, while sodium has only one stable isotope, Na-23.
Gold is the most malleable metal, which can be made into sheets as thin as 1/10000mm. Platinum is the most ductile metal, which can be drawn into wires with a diameter as thin as 1/5000mm.
Tungsten has the highest melting point of all metals, with a melting temperature of 3410℃, while mercury has the lowest melting point, melting at -38.8℃. Gallium has the largest difference between its melting point (30℃) and boiling point (2403℃). Francium has the lowest concentration in the Earth’s crust, with a content of only 37 per ton × 10-13g, or about 1 × 10-21%.
Cesium is the most responsive metal to light and generates the greatest current. When its surface is illuminated, electrons can gain energy and escape the surface, resulting in a photoelectric current. Cesium also has the greatest metallic properties of all metals.
Californium is the most expensive metal in the world, with a price of US $10 million per gram, making it over 500,000 times more expensive than gold. Iron is the least expensive metal.
Niobium is the most practical superconducting element, becoming a superconductor with almost no resistance when cooled to -263.9℃. Palladium has the greatest ability to absorb gas, with one volume of colloidal palladium able to absorb up to 1,200 volumes of hydrogen.
It is not always possible to tell if a metal is toxic just by looking at it or handling it. Some metals can be toxic in certain forms or at certain concentrations, while others may not be toxic at all.
The toxicity of a metal depends on factors such as the form of the metal (e.g. solid, liquid, gas), the concentration or dose of exposure, and the route of exposure (e.g. inhalation, ingestion, skin contact).
To determine the toxicity of a metal, it is important to consult reliable sources such as the Material Safety Data Sheet (MSDS) or other safety guidelines. These sources will provide information on the hazards and precautions associated with the metal, as well as guidelines for safe handling, storage, and disposal.
It is also important to follow proper safety procedures when handling any metal, such as wearing protective equipment like gloves, goggles, and respirators, and avoiding direct contact with the metal whenever possible.
Out of 118 known elements in the periodic table, about 90 are considered metals. The exact number depends on how you classify some borderline elements (with both metal and nonmetal characteristics).