What makes a material suitable for a specific engineering application? The answer lies in understanding its properties. This article covers 11 essential material properties, such as mechanical strength, impact toughness, and thermal conductivity, offering insights into their definitions, significance, and practical implications. By the end, you’ll grasp how these properties influence material performance and their critical role in engineering design and manufacturing.
Craze: Craze is a type of defect that occurs in the deformation process of polymer materials. It appears as a silver color due to its low density and high reflectiveness to light. Craze occurs in the weak or defective parts of polymer materials.
Superplasticity: Under certain conditions, the material exhibits a very large elongation (around 1000%) without necking or breaking, which is called superplasticity. The proportion of strain generated by grain boundary sliding, εg, in total strain, εt, is typically between 50% and 70%, indicating that grain boundary sliding plays a major role in superplastic deformation.
Brittle Fracture: Before the material fractures, there is no obvious macroscopic plastic deformation, and no warning signs. This process is often sudden and rapid, making it very dangerous.
Ductile Fracture: The fracture process that shows obvious macroscopic plastic deformation before and during the fracture. In ductile fracture, the crack propagation process is generally slow and consumes a large amount of plastic deformation energy.
Cleavage Fracture: The brittle fracture along a specific crystal plane caused by the destruction of bonding bonds between atoms under normal stress is called cleavage fracture. The cleavage step, river pattern, and tongue pattern are the basic microscopic characteristics of cleavage fracture.
Shear Fracture: Shear fracture is the fracture caused by material sliding and separation along the slip plane under shear stress. Micropore aggregation fracture is a common mode of ductile fracture in materials. The fracture surface is usually dark gray and fibrous in macro, while the micro fracture surface has a characteristic pattern of many “dimples” distributed on the surface.
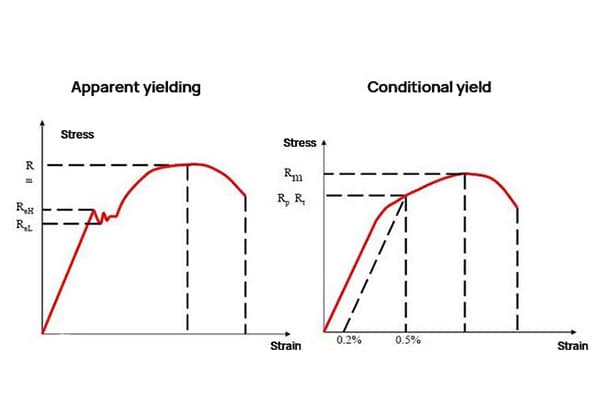
Type of stress, degree of plastic deformation, presence or absence of omen, and speed of crack propagation.
If the material experiences no plastic deformation or very little plastic deformation before fracture, and brittle fracture occurs without necking, then the critical stress, σc, is equal to the breaking stress, σb.
However, if necking occurs before fracture, σc and σb are not equal.
The Griffith formula is only appropriate for brittle solids containing microcracks, such as glass, inorganic crystal materials, and ultra-high strength steel.
For many engineering structural materials, such as structural steel and polymer materials, the crack tip undergoes significant plastic deformation, which consumes a large amount of plastic deformation energy.
Therefore, the Griffith formula must be modified to accurately reflect this phenomenon.
The ratio of the maximum shear stress, τmax, to the maximum normal stress, σmax, is called the stress state softness coefficient, denoted by α.
The larger α is, the larger the maximum shear stress component becomes, indicating a softer stress state and greater ease of plastic deformation in the material.
Conversely, the smaller α is, the harder the stress state becomes, leading to more brittle fracture.
When a specimen has a notch, its yield stress is higher than that of a specimen under uniaxial tension due to the presence of triaxial stress, which is referred to as the “notch strengthening” phenomenon.
However, this “notch strengthening” cannot be considered a method of material strengthening, as it is solely a result of the plastic deformation of the material constrained by three-dimensional stress.
In this case, the material’s own value of σs remains unchanged.
Revised:
In unidirectional tension, the normal stress component is large while the shear stress component is small, resulting in a hard stress state.
This test is typically applied to materials with low plastic deformation resistance and cutting resistance, known as plastic materials.
Unidirectional compression has a stress state softness coefficient of a=2, and is mainly used to test brittle materials.
Bending tests do not suffer from the specimen deflection that occurs during tension tests.
In bending, the stress distribution on the cross section reaches its maximum at the surface, making it an effective way to reflect surface defects in materials.
Torsion Test: The softness coefficient of stress state in torsion is higher than that in tension, making it an effective method for evaluating the strength and plasticity of materials that are brittle under tension.
In the torsion test, the stress distribution on the sample section is highest at the surface, making it highly sensitive to the material’s surface hardening and surface defects.
The torsion test results in approximately equal normal stress and shear stress.
The fracture surface in the torsion test is perpendicular to the sample axis and is often used to evaluate plastic materials.
In normal fracture, the angle between the fracture surface and the sample axis is approximately 45 degrees, which is due to normal stress. Brittle materials frequently exhibit this type of fracture surface.
The principle of the Vickers hardness test is similar to the Brinell hardness test, as both methods calculate hardness values based on the load per unit area of the indentation.
The main difference between the two tests is the type of indenter used. In the Vickers hardness test, a diamond pyramid indenter with an angle of 136 degrees between opposite sides is employed. In contrast, the Brinell hardness test uses a hardened steel ball or a hard alloy ball as the indenter.
Advantages of Brinell hardness test:
The large indentation area of the Brinell hardness test makes it capable of reflecting the average performance of each constituent phase over a large area, and the test results are stable and highly repeatable.
As a result, the Brinell hardness test is particularly suitable for measuring the hardness of materials such as gray cast iron and bearing alloys.
Disadvantages of Brinell hardness test:
The large indentation diameter of the Brinell hardness test makes it generally unsuitable for direct inspection of finished products.
Furthermore, the need to replace the indenter diameter and load for materials with varying hardness, as well as the inconvenience of measuring the indentation diameter, are additional drawbacks of the test.
Advantages of Rockwell hardness test:
Easy and fast operation;
The indentation is small, and the workpiece can be inspected directly;
Disadvantages:
Poor representation due to small indentation;
The hardness values measured with different scales can neither be directly compared nor exchanged.
The Vickers hardness test has many advantages:
Accurate and reliable measurement;
You can select any load.
In addition, Vickers hardness does not have the problem that the hardness of different scales of Rockwell hardness cannot be unified, and the thickness of the test piece is thinner than that of Rockwell hardness.
Disadvantages of Vickers hardness test:
Its measuring method is troublesome, its working efficiency is low, the indentation area is small, and its representativeness is poor, so it is not suitable for routine inspection of mass production.
Related reading: Metal Hardness: The Definite Guide
When the temperature during testing drops below a certain temperature, tk (the ductile-brittle transition temperature), materials such as bcc or closely packed hexagonal crystal metals and alloys, particularly medium and low strength structural steels commonly used in engineering, undergo a transition from a ductile state to a brittle state, resulting in a significant decrease in impact absorption energy.
This transition is characterized by a change in fracture mode from micropore aggregation to transgranular cleavage and a change in fracture appearance from fibrous to crystalline, a phenomenon known as low-temperature brittleness.
At temperatures below the ductile-brittle transition temperature, the fracture strength is lower than the yield strength, resulting in brittle behavior at low temperatures.
A. Influence of crystal structure: Body centered cubic metals and their alloys have low temperature brittleness, while face centered cubic metals and their alloys generally have no low temperature brittleness.
The low temperature brittleness of BCC metals may be closely related to the late yielding phenomenon.
B. The influence of chemical composition: the content of interstitial solute elements increases, the higher energy decreases, and the ductile brittle transition temperature increases.
C. Influence of microstructure: refining grain and structure can increase the toughness of materials.
D. Influence of temperature: It is relatively complex, and brittle (blue brittle) occurs within a certain temperature range.
E. Effect of loading rate: Increasing the loading rate is like lowering the temperature, which increases the brittleness of the material and increases the ductile brittle transition temperature.
F. Influence of specimen shape and size: the smaller the curvature radius of notch, the higher tk.
Grain boundaries serve as resistance to crack propagation.
The reduction in the number of dislocations at the pre-grain boundary packing helps to reduce stress concentration.
An increase in the total grain boundary area reduces impurity concentration along the grain boundaries, thereby reducing the likelihood of intergranular brittle fracture.
When the working stress of large parts is not high, even far below the yield limit, brittle fracture often occurs, which is called low stress brittle fracture.
KIC (the stress-strain field intensity factor at the crack tip in the crack body) is a measure of plane strain fracture toughness and represents a material’s ability to resist unstable crack propagation under plane strain conditions.
JIc (the strain energy at the crack tip) is also known as fracture toughness and represents a material’s ability to resist crack initiation and propagation.
GIc represents the energy consumed per unit area to prevent unstable crack propagation in a material.
δC (crack opening displacement), also known as material fracture toughness, indicates a material’s ability to prevent crack expansion from starting.
KI and KIC are two distinct concepts.
KI is a mechanical parameter that represents the strength of the stress-strain field at the crack tip in the crack body and is dependent on applied stress, sample size, and crack type, but is independent of the material.
On the other hand, KIC is a mechanical property index of the material that depends on internal factors such as composition and structure, but is not affected by external factors like applied stress and sample size.
The relationship between KI and KIC is similar to that between σ and σs, where KI and σ are mechanical parameters, and KIC and σs are mechanical property indexes of materials.
(1) This type of failure is a sudden and unexpected failure that occurs without noticeable plastic deformation before fatigue failure and is characterized by brittle fracture.
(2) Fatigue failure is a type of low stress cycle delayed fracture.
(3) Fatigue is highly sensitive to defects such as notches, cracks, and structural defects.
(4) Fatigue forms can be classified in several ways.
According to stress state, fatigue forms include bending fatigue, torsion fatigue, tension and compression fatigue, contact fatigue, and composite fatigue.
Based on stress level and fracture life, fatigue can be further classified into high cycle fatigue and low cycle fatigue.
Fatigue source, fatigue crack growth zone and transient fracture zone.
σ-1 (fatigue strength) represents the infinite life fatigue strength of smooth specimens, which is suitable for traditional fatigue strength design and verification;
ΔKth (threshold value of fatigue crack growth) represents the infinite life fatigue performance of the crack sample, which is suitable for the design and fatigue strength check of the cracked parts.
Adhesion wear, abrasive wear, corrosion wear and pitting fatigue wear (contact fatigue).
Adhesion wear: The wear surface is characterized by scabs of different sizes on the surface of the parts.
Abrasive wear: groove formed by scratch or obvious furrow on friction surface.
Contact fatigue: there are many pits (pockmarks) on the contact surface, some of which are deep, and there are traces of fatigue crack growth lines at the bottom.
Correct. Because the wear is inversely proportional to the hardness.
The residual compressive stress of the surface layer is increased while the surface strength and hardness are increased.
Approximate specific temperature: T/Tm
Creep: This refers to the gradual plastic deformation of a material under the influence of constant temperature and load over an extended period of time.
Endurance Strength: This term refers to the maximum stress that a material can withstand without experiencing creep fracture, under a specific temperature and within a designated time frame.
Creep Limit: This represents a material’s resistance to high-temperature creep deformation.
Relaxation Stability: The term used to describe a material’s ability to resist stress relaxation is called relaxation stability.
The main mechanisms of creep deformation in materials include dislocation slip, atomic diffusion, and grain boundary slip.
For polymer materials, molecular chain stretching under external force is also a contributing factor to creep.
Intercrystalline fracture is a common form of creep fracture, particularly at high temperatures and low stress levels. This is because the strength of polycrystalline grains and grain boundaries decreases with temperature, but the latter decreases faster, leading to lower grain boundary strength relative to grain strength at high temperatures.
There are two models to explain grain boundary fracture: the grain boundary sliding and stress concentration model, and the vacancy aggregation model.
The plastic deformation mechanism of metals is based on slip and twinning.
The creep deformation mechanism of metals is primarily driven by dislocation slip, diffusion creep, and grain boundary slip.
At high temperatures, the elevated temperature provides thermal activation for atoms and vacancies, allowing dislocations to move and continue to cause creep deformation.
Under the influence of external force, an uneven stress field is generated within the crystal, leading to differences in potential energy among atoms and vacancies. This results in directional diffusion from high potential energy to low potential energy.
For solid materials, the heat capacity is not significantly impacted by the material’s structure.
In a first-order phase transition, the heat capacity curve changes abruptly and has an infinite value.
In a second-order phase transformation, the change occurs gradually over a specific temperature range and results in a finite maximum heat capacity.
Amorphous materials have low thermal conductivity because their short-range ordered structure can be considered as a crystal with extremely small grains.
Due to the small grain size and numerous grain boundaries, phonons are easily scattered, leading to significantly reduced thermal conductivity.
Under the action of magnetic field, the orbital motion of electrons in matter produces diamagnetism.
Determining the Maximum Solubility Curve in the Alloy Phase Diagram:
By utilizing the rule that single-phase solid solutions exhibit higher paramagnetism than two-phase mixed structures and the linear relationship between mixture paramagnetism and alloy composition, the maximum solubility of an alloy at a specific temperature and the alloy solubility curve can be determined.
Investigating the Decomposition of Aluminum Alloys:
The order-disorder transition, isomerism transition, and recrystallization temperature were studied to better understand the decomposition of aluminum alloys.
For a metal to exhibit ferromagnetism, it is not only necessary for its atoms to have non-zero spin magnetic moments, but also for these moments to spontaneously align and generate spontaneous magnetization.
Soft magnetic materials have a narrow hysteresis loop and are characterized by high magnetic conductivity and low Hc. In contrast, hard magnetic materials have a thick hysteresis loop, high Hc, Br, and (BH)m.
In a metal, the electric field created by positive ions is uniform and there is no interaction between the valence electrons and ions. This field is considered a property of the entire metal and allows for free movement of electrons throughout the metal.
According to the quantum free electron theory, the inner electrons of each atom in the metal retain the energy state of a single atom, while the valence electrons have different energy states due to quantization and have distinct energy levels.
The energy band theory also recognizes that the valence electrons in metals are shared and quantized in energy, but it suggests that the potential field created by ions in metals is not uniform, but instead changes periodically.
The rise in temperature intensifies ion vibration and increases the amplitude of thermal vibration, leading to increased atomic disorder, reduced electron movement, and increased scattering probability. These factors result in an increase in resistivity.
In semiconductors, conduction is mainly driven by electrons and holes. An increase in temperature increases the kinetic energy of electrons, leading to an increase in the number of free electrons and holes in the crystal, and thus an increase in conductivity and a decrease in resistance.
(1) Critical transition temperature Tc
(2) Critical magnetic field Hc
(3) Critical current density Jc
The change of microstructure of metals and alloys is studied by measuring the change of resistivity.
(1) Measure the solubility curve of solid solution
(2) Measure the transformation temperature in shape memory alloy.
Thermal effect, photosensitive effect, pressure sensitive effect (voltage sensitive and pressure sensitive), magnetic sensitive effect (Hall effect and magnetoresistance effect), etc.
Electrical breakdown, thermal breakdown and chemical breakdown.
Linear Optical Properties: When light of a single frequency is incident on a transparent medium that does not absorb light, its frequency does not change. When light of different frequencies is incident on the medium at the same time, there is no interaction between the light waves and no new frequency is produced.
When two beams of light intersect, if they are coherent light, interference will occur. If they are incoherent light, only the intensity of the light will combine, following the principle of linear superposition.
Other optical properties include refraction, dispersion, reflection, absorption, and scattering.
It is not practical to use metals for visible light optics because they strongly absorb visible light. This is because the valence electrons in metals occupy an incomplete band and, after absorbing photons, are in an excited state. They can transfer energy through collisions and produce heat, but do not transition to the conduction band.
The incident light is strong;
Symmetry requirements of crystals;
Phase matching.