Have you ever wondered about the secret behind the strength and durability of titanium alloys? In this article, we’ll dive into the fascinating world of heat treatment techniques that transform these alloys into high-performance materials. Our expert team will guide you through the principles, processes, and effects of various heat treatment methods, providing valuable insights for engineers and enthusiasts alike. Get ready to uncover the science behind the remarkable properties of titanium alloys!
(1) The martensitic phase transformation does not cause significant changes in the properties of titanium alloys. This feature is different from the martensitic phase transformation of steel. Heat treatment strengthening of titanium alloys relies on aging decomposition of the sub-stable phase formed by quenching, including the martensitic phase. Heat treatment for pure a-type titanium alloys is basically ineffective; it is mainly used for α+β-type titanium alloys.
(2) Heat treatment should avoid the formation of the ω phase as it makes titanium alloys brittle. The correct choice of the aging process can make the ω phase decompose, such as using a higher aging temperature.
(3) It is difficult to refine titanium alloy grains using repeated phase transformations, unlike steel materials. Repeated phase transformation of austenite and pearlite (or ferrite, cementite) can control the nucleation and growth of new phases to achieve grain refinement in most steels. This phenomenon does not exist in titanium alloys.
(4) Poor thermal conductivity can lead to poor hardenability of titanium alloys, especially α+β titanium alloys. The quenching thermal stress is large, and the parts are prone to warping during quenching. Due to poor thermal conductivity, deformation of the titanium alloy easily causes local temperature rise, which may cause the local temperature to exceed the β transformation point and form the Widmanstatten structure.
(5) Lively chemical properties make titanium alloys easily react with oxygen and water vapor during heat treatment. It forms an oxygen-rich layer or scale on the surface of the workpiece, reducing the alloy’s performance. At the same time, titanium alloys tend to absorb hydrogen during heat treatment, causing hydrogen embrittlement.
(6) The β transition point varies significantly, even if it is the same composition, due to different smelting furnaces.
(7) When heating in the β phase region, β grains tend to grow larger. The coarsening of β grains can make the alloy’s plasticity drop sharply, so the heating temperature and time should be strictly controlled. Heat treatment for heating in the β phase region should be used with caution.
The phase transformation of titanium alloy is the basis of titanium alloy heat treatment. To improve the performance of titanium alloys, it is necessary to use appropriate heat treatment in addition to reasonable alloying.
There are many types of heat treatments for titanium alloys, including annealing treatment, aging treatment, deformation heat treatment, and chemical heat treatment, among others.
Annealing is suitable for various titanium alloys, and its main purpose is to eliminate stress, improve alloy plasticity, and stabilize the structure.
The forms of annealing include stress relief annealing, recrystallization annealing, double annealing, isothermal annealing, vacuum annealing, among others.
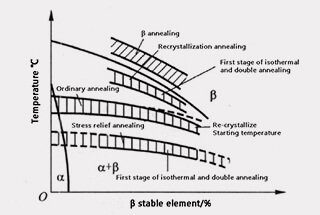
Figure 1 shows the annealing temperature range of titanium alloy using various methods.
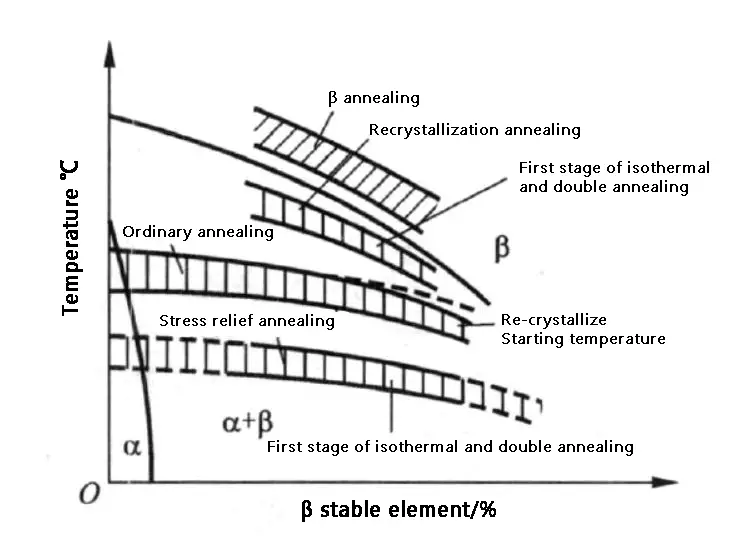
Figure 1 Schematic diagram of the annealing temperature range of various methods in titanium alloy
(1) Stress relieving annealing.
Stress relieving annealing can be used to eliminate internal stress generated during casting, cold deformation, and welding.
The temperature for stress relieving annealing should be lower than the recrystallization temperature, typically between 450-650 ℃.
The required time depends on the workpiece’s cross-sectional size, processing history, and the degree of stress relief required.
(2) Ordinary annealing.
The purpose of ordinary annealing is to eliminate basic stresses in the titanium alloy semi-finished product and increase strength and plasticity in accordance with the required technical conditions.
The annealing temperature and recrystallization temperature are typically equivalent to or slightly lower than the start temperature. This annealing process is generally used in factory metallurgical products and can also be called factory annealing.
(3) Complete annealing.
The purpose of complete annealing is to completely eliminate process hardening, stabilize the organization, and improve plasticity. This process occurs mainly through recrystallization and is also known as recrystallization annealing.
The annealing temperature is preferably between the recrystallization temperature and the phase transition temperature. If the temperature exceeds the phase transition temperature, the Widmanstatten structure will form, and the alloy’s properties will deteriorate.
The type of annealing, temperature, and cooling methods are different for various kinds of titanium alloys.
(4) Double annealing.
Double annealing can be used to improve the plasticity of the alloy, fracture toughness, and stability of the organization. After annealing, the alloy organization is more uniform and close to the equilibrium state.
This type of annealing is often used to ensure the stability of the structure and performance of heat-resistant titanium alloys under high temperature and long-term stress.
Double annealing involves heating and air-cooling the alloy twice. The heating temperature of the first high-temperature annealing is higher than or close to the end temperature of recrystallization so that recrystallization can proceed fully without significant crystal grain growth, and the volume fraction of the ap phase is controlled.
The structure is not stable enough after air-cooling, and a second low-temperature annealing is required. The annealing temperature is lower than the recrystallization temperature, and the temperature is kept for a long time to fully decompose the metastable β phase obtained by high-temperature annealing.
(5) Isothermal annealing.
Isothermal annealing can obtain the best plasticity and thermal stability and is suitable for dual-phase titanium alloys with a high content of β-stabilizing elements.
Isothermal annealing adopts hierarchical cooling, which means that after heating to a temperature above the recrystallization temperature, the workpiece is immediately transferred to another lower temperature furnace (generally 600-650℃) for insulation, and then air-cooled to room temperature.
Quenching aging is the main way to strengthen titanium alloy heat treatment, using phase change to produce a strengthening effect, which is also known as heat treatment strengthening.
The strengthening effect of titanium alloy heat treatment is determined by the nature of the alloy element, concentration, and heat treatment specifications.
These factors affect the type, composition, quantity, and distribution of the metastable phase obtained by alloy quenching, as well as the nature, structure, and dispersivity of the precipitated phase during the decomposition of the metastable phase, which is related to the alloy composition, heat treatment process specifications, and original structure.
For alloys with a certain composition, the effect of aging strengthening depends on the selected heat treatment process.
The higher the quenching temperature, the more obvious the effect of aging strengthening, but quenching above the β transformation temperature will cause brittleness due to excessively coarse grains.
For two-phase titanium alloys with lower concentration, higher temperature quenching can be used to obtain more martensite.
Two-phase titanium alloys with a higher concentration should be quenched at a lower temperature to obtain more metastable β-phase, so that the maximum aging strengthening effect can be obtained.
The cooling method is generally water-cooled or oil-cooled, and the quenching process should be rapid to prevent the decomposition of the β phase during the transfer process and reduce the aging strengthening effect.
The aging temperature and time should be chosen to obtain the best overall performance criteria, with a general aging temperature of 500-600℃ in α + β-type titanium alloy and a time of 4-12 hours.
The aging temperature of β-type titanium alloy is 450-550℃, time is 8-24 hours, and the cooling method is air-cooling.
Deformation heat treatment is an effective combination of pressure processing (forging, rolling, etc.) and heat treatment technology, which allows for both deformation strengthening and heat treatment strengthening to obtain an organization and comprehensive performance that cannot be achieved with a single strengthening method.
A common deformation heat treatment process is shown in Figure 2.
Different types of thermomechanical heat treatment are classified according to the relationship between the deformation temperature and the recrystallization temperature and phase transition temperature.
According to the deformation temperature, it can be divided into:
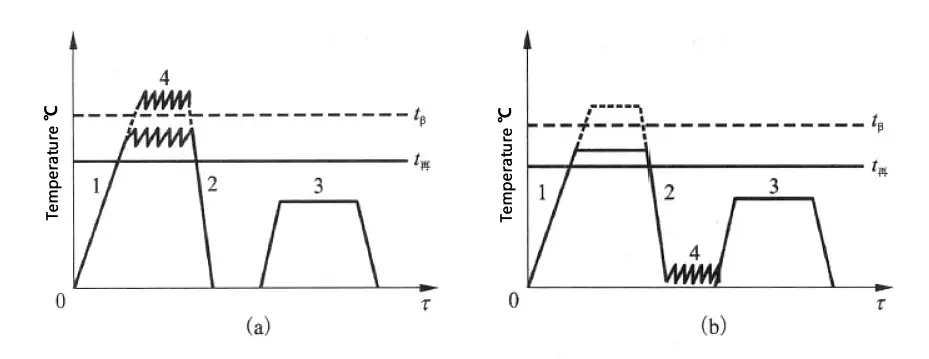
Figure 2 Schematic representation of the deformation heat treatment process in titanium alloy.
(a) High-temperature deformation heat treatment;
(b) Low-temperature deformation heat treatment
(1) High-temperature thermomechanical treatment
It involves heating above the recrystallization temperature, deforming by 40% to 85%, then rapidly quenching, and then subjecting to conventional aging heat treatment.
(2) Low-temperature thermomechanical treatment
Deformation is done about 50% below the recrystallization temperature, followed by conventional aging treatment.
(3) Compound thermomechanical treatment
It is a process that combines high-temperature thermomechanical treatment and low-temperature thermomechanical treatment.
Titanium alloys have a high friction coefficient and poor wear resistance (generally about 40% lower than steel), making them prone to adhesion on contact surfaces and causing frictional corrosion.
Titanium alloys are more resistant to corrosion in oxidizing media but less resistant to corrosion in reducing media (such as hydrochloric acid, sulfuric acid, etc.).
To improve these properties, electroplating, spraying, and chemical heat treatment (such as nitriding, oxygenation, etc.) can be used.
The hardness of the nitrided layer after nitriding is 2 to 4 times higher than that of the surface layer without nitriding, significantly improving the wear resistance of the alloy, while also improving the corrosion resistance of the alloy in reducing media.
Oxygen infiltration can increase the corrosion resistance of the alloy by 7-9 times, but the plasticity and fatigue strength of the alloy will be lost to different extents.
Microstructure characteristics of titanium alloy
In titanium alloys, especially α+β duplex titanium alloys, a wide variety of structures can be observed.
These structures differ in morphology, grain size, and intragranular structure, mainly depending on alloy composition, deformation process, and heat treatment process.
In general, titanium alloys have two basic phases: α-phase and β-phase.
The mechanical properties of titanium alloys largely depend on the proportion, morphology, size, and distribution of these two phases.
The structural types of titanium alloys can be basically divided into four categories: Widmanstatten structure (lamellar structure), basketweave structure, bimodal structure, and isometric structure.
Figure 3 shows the typical morphological characteristics of the various types of titanium alloys.
Table 1 provides the alloy performance indices of TC4 titanium alloy in four typical structural states, which show that the performance of different structures varies greatly.
Table 1: Influence of four typical tissues on the performance in TC4 alloy
| Mechanical property | Compressive strength σ /MPa | Elongation δ /% | Impact toughness αk /(KJ*m-2) | Fracture toughness KIC /(MPa*m1/2) |
| lamellar structure | 1020 | 9.5 | 355.3 | 102 |
| basketweave structure | 1010 | 13.5 | 533 | ___ |
| bimodal structure | 980 | 13 | 434.3 | ___ |
| isometric structure | 961 | 16.5 | 473.8 | 58.9 |
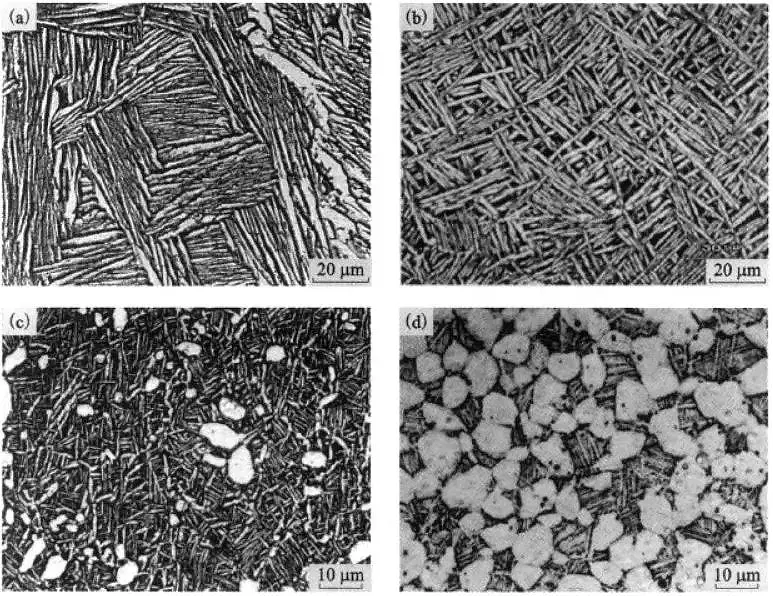
Figure 3 Typical organization in titanium alloys
(a) lamellar tissue; (b) basketweave tissue; (c) bimodal tissue; (d) isometric tissue
Lamellar tissue
It is characterized by coarse original β crystal grains and complete grain boundary α phase, forming large-sized “bundles” in the original β crystal grains, and there are more in the same “bundles”. The slices are parallel to each other and in the same orientation, as shown in Figure 3(a).
This kind of microstructure is the structure formed when the alloy is not deformed or deformed after heating in the beta phase region, and it is slowly cooled down from the beta phase region. When the alloy has this structure, its fracture toughness, durability and creep strength are good, but its plasticity, fatigue strength, notch sensitivity, thermal stability, and thermal stress corrosion resistance are very poor. These properties vary with the size of the α “bundle” and the thickness of the grain boundary α. The α “bundle” becomes smaller, the grain boundary α becomes thinner, and the overall performance is improved.
Basketweave tissue
Its characteristic is that the original β grain boundary is destroyed during the deformation process, and no or only a small amount of dispersed granular grain boundary α appears, and the α slices in the original β grain become shorter.
The size of α “bundle” is small, and the clusters are arranged staggered, like a woven basket, as shown in Figure 3(b).
This kind of microstructure is generally formed when the alloy is heated or begins to deform in the β-phase region or the amount of deformation in the (α+β) dual-phase region is not large enough.
The fine mesh basket structure not only has better plasticity, impact toughness, fracture toughness, and high cycle fatigue strength but also has better thermal strength.
Bimodal tissue
Its characteristic is that unconnected primary α is distributed on the matrix of p-transformation tissue, but the total content does not exceed 50%, as shown in Figure 3(c).
When the heating temperature of thermal deformation or heat treatment of the titanium alloy is less than the β transformation temperature, a dual-state structure can generally be obtained.
Bimodal structure refers to the α-phase in the organization having two forms: one is the primary equiaxed α-phase, and the other is the lamellar α-phase in the β-transformed organization, which corresponds to the primary α-phase.
The phase is also called the secondary α-phase or secondary α-phase.
This structure is formed when the alloy is at a higher temperature and greater deformation in the (α+β) dual-phase zone.
Isometric tissue
Its characteristic is that a certain amount of transformed β structure is distributed on the primary α-phase matrix with a uniformly distributed content of more than 50%, as shown in Figure 3(d).
The deformation processing and heat treatment of the titanium alloy are all carried out in the (α+β) dual-phase zone or the α-phase zone, and when the heating temperature is much lower than the β transformation temperature, an equiaxed structure can generally be obtained.
Compared with other structures, this structure has better plasticity, fatigue strength, and thermal stability, but its fracture toughness, durability, and creep strength are worse.
Because this structure has better overall performance, it is currently the most widely used.
Effect of Heat Treatment Process on Microstructure Evolution of Titanium Alloy
The heat treatment process for titanium alloys is shown in Figure 4.
The main parameters that are controlled during the process are the solid solution temperature, solid solution time, cooling method (including water quenching, oil quenching, and air cooling), furnace cooling, aging temperature, and aging time.
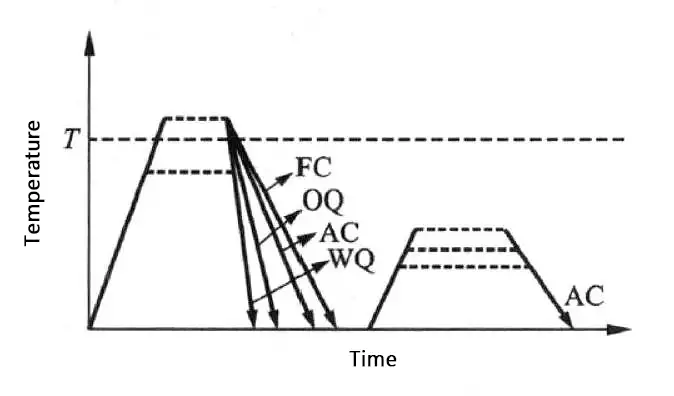
Figure 4 Process diagram of a typical heat treatment
Effect of solid solution temperature on the microstructure of TC21 alloy
Figure 5 shows the microstructure of TC21 alloy at different solid solution temperatures.
It can be seen that as the solid solution temperature increases, the volume fraction of the αp phase decreases.
When the solid solution temperature is higher than Tβ, the αp phase disappears.
During the solution treatment at 940°C, due to the obstruction of the equiaxed αp phase, the grain boundaries of the β grains bend and bow, as shown by the arrow in Figure 5(c).
When a solution treatment is applied at a temperature higher than Tβ (i.e., 1000°C), the αp phase disappears.
As the obstacles to the movement of the β grain boundaries disappear, the β grains grow sharply, with an average diameter of about 300 μm, as shown in Figure 5(d).
It can be seen that the solution temperature has a significant effect on the microstructure of TC21 alloy.
When the (α+β) dual-phase region is solid solution treated, the size, morphology and distribution of αp phase will directly affect the size of β crystal grains.
The αp phase and β grain size of the titanium alloy play a vital role in the mechanical properties of the alloy.
To avoid the rapid growth of β grains, the solid solution temperature of TC21 alloy should be selected below Tβ, so that a relatively suitable grain size can be obtained, and a dual-state structure composed of primary and secondary phases can be obtained.
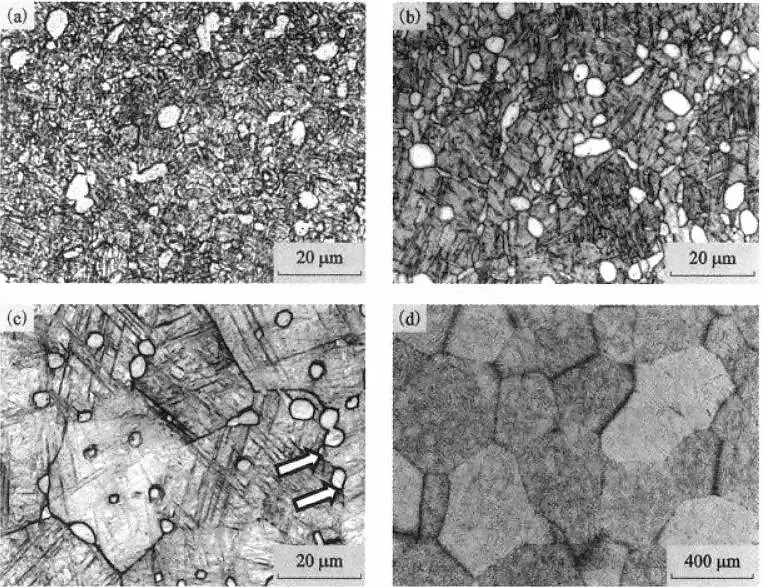
Figure 5 The effect of solution temperature on the microstructure of TC21 alloy
(a)850℃/AC; (b)910℃/AC; (c)940℃/AC; (d)1000℃/AC
Effect of Solution Time on Microstructure of TC21 Alloy
Figure 6 shows the microstructure of TCIZ alloy after solution treatment and air cooling for 4 hours.
From Figures 6, 5(a), and 5(b), it can be seen that the volume fraction and distribution of the ap phase in the TC21 alloy do not change significantly with an increase in the solution time.
It is apparent that when the solution treatment reaches a certain time, the microstructure of TC21 alloy is not sensitive to the solution treatment time, but the solution treatment temperature plays a decisive role in the solid solution structure of the alloy.
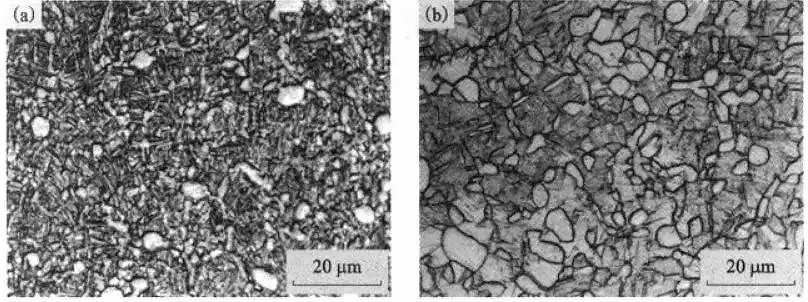
Figure 6 The effect of solution time on the microstructure of TC21 alloy
(a)850℃/4h, AC; (b)910℃/4h, AC
Effect of Cooling Method on Microstructure of TC21 Alloy
Figure 7 shows the effect of cooling methods on the microstructure of TC21 alloy.
It can be seen that the cooling method has a significant effect on the microstructure of TC21 alloy after solution treatment.
Under WQ and OQ conditions, due to the faster cooling rate, only metastable β is formed, but no βT is formed.
Under AC conditions, a certain amount of βT is formed.
The size of the αp phase obtained under WQ and OQ conditions is slightly smaller than that obtained under AC conditions.
This difference is due to the slow cooling rate of AC, allowing the αp phase in the alloy to fully grow during the cooling process (causing the αp phase content in the alloy to increase and aggregate growth under AC conditions).
In the slower cooling process, the β phase at high temperature can also be fully transformed to form βT.
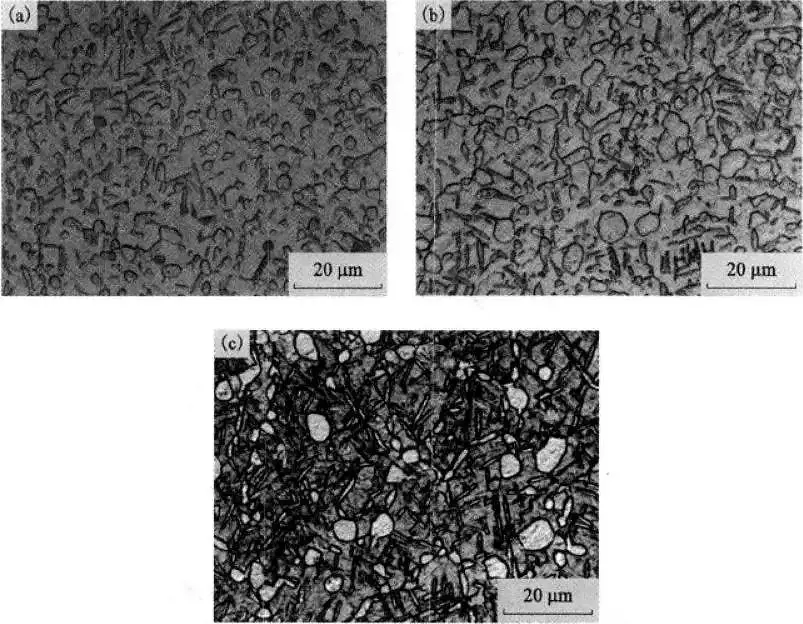
Figure 7 The effect of the cooling method on the microstructure of TC21 alloy
(a)910℃/1h, WQ; (b)910℃/1h, OQ; (c)910℃/1h, AC
Effect of Aging Temperature on the Structure of TC21 Alloy
Figure 8 shows a microstructure photograph of TC21 alloy aged at 500°C and 600°C.
It is evident from Figure 8 that the structure of the alloy after aging is composed of αp phase and βT phase.
As aging proceeds, the secondary α-phase grows and merges.
The secondary α-phase gradually increases with increasing aging temperature.
As depicted in Figure 8(a), (b), and (c), at 500 °C aging, due to the low aging temperature, the sub-stable β obtained from the solid solution treatment lacks the driving force for decomposition during the aging process, and thus fewer secondary phases are formed.
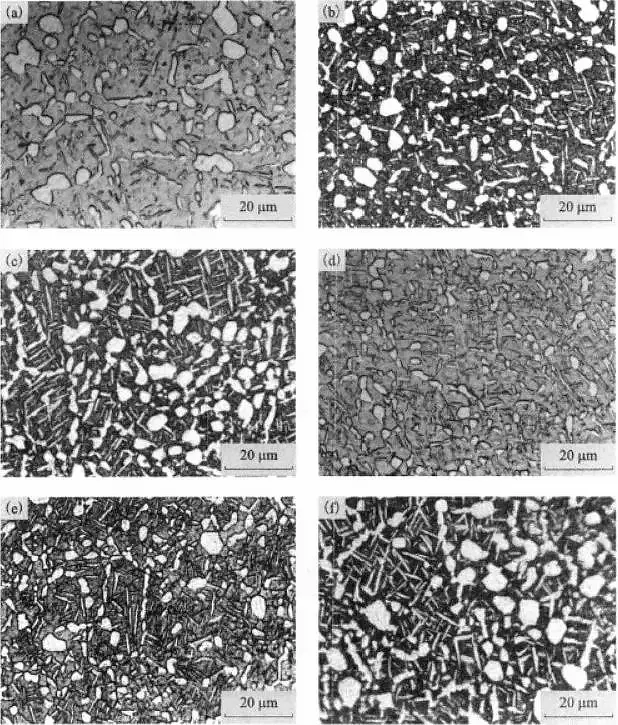
Figure 8 Effect of aging temperature on the structure of TC21 alloy
Effect of Aging Time on the Structure of TC21 Alloy
Figure 9 shows microstructure photos of TC12 alloy aged at 550°C for different times.
It can be observed from Figure 9 that with the increase in duration of aging, the volume fraction of βT phase increases, while the size of the αp phase does not change significantly, but agglomeration and growth phenomena occur.
The larger secondary strip-like α phases also appear to merge and grow.
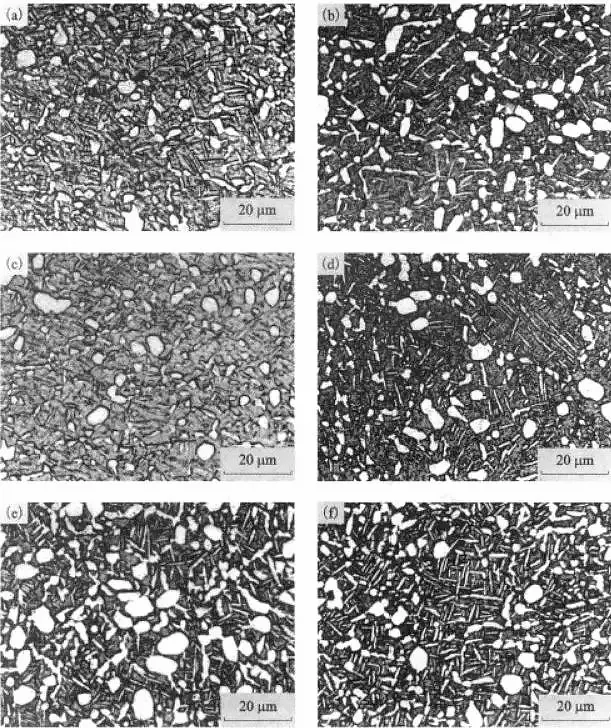
Figure 9 The effect of aging time on the structure of TC21 alloy
Effect of Heat Treatment on the Microstructure of Typical Titanium Alloy
By controlling the heat treatment process conditions of TC12 alloy and Ti60 alloy, two major types of lamellar microstructure and bimodal microstructure are obtained, as shown in Figure 10.
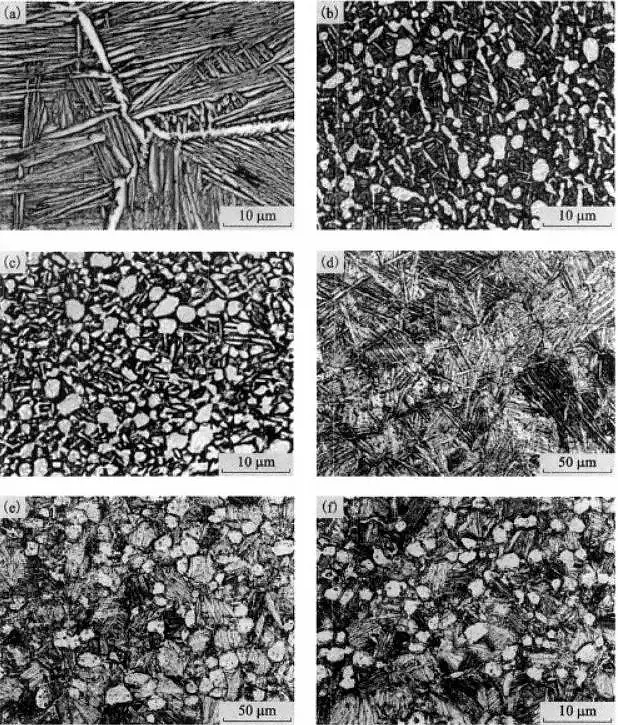
Figure 10 The effect of heat treatment on the microstructure of a typical titanium alloy
Figure 10 shows that the Ti600 alloy can obtain LM and BM structures by selecting the solid solution temperature above and below Tb (1010°C), respectively.
The thickness of lamella in LM tissue is 2-3 μm, and the volume fraction of αp phase in BM tissue is about 20%, with an average diameter of about 15 μm.
Figure 10(f) displays the microstructure of Ti600 alloy with BM structure after 100h thermal exposure (TE) at 600℃.
The differences between BM and BM+TE tissues cannot be distinguished from the microscopic tissues shown in Figure 10(e) and (f) alone.
Al-rich αp phase in high-temperature titanium alloys is prone to precipitate the α2 (Ti3Al) phase during long-term aging or thermal exposure.
By transmission electron microscopy, the α2 phase was found in the αp phase of BM tissue Ti600 alloy after thermal exposure, as shown in Figure 11.
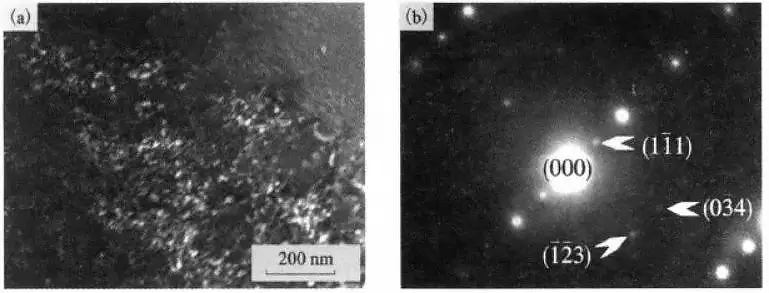
Figure 11 TEM morphology and selected area electron diffraction pattern of α2 phase in Ti600 alloy after thermal exposure
(a) TEM topography; (b) selected area electron diffraction pattern