Have you ever wondered why some metals last longer than others? In this article, we’ll explore the fascinating world of corrosion resistance in metals and alloys. You’ll learn how different materials react in various environments and discover the best ways to protect metal equipment from rust and decay. Get ready to uncover the secrets behind durable engineering!

The selection of corrosion-resistant materials is the most effective and proactive measure to ensure the reliable operation of metal equipment.
Therefore, it is necessary to have a grasp of the corrosion resistance of various metals and alloys, understand the appropriate working environment for each material, and only in this way can effective anti-corrosion measures be taken for the corrosion of metal equipment.
“Iron-based alloys (steel and cast iron) are the most commonly used metal materials in engineering and have satisfactory corrosion resistance and good comprehensive mechanical properties in certain situations. Its corrosion resistance is closely related to the corrosion resistance of pure iron.

Iron is a thermodynamically unstable metal and has poor corrosion resistance compared to metals close to its equilibrium potential, such as aluminum, titanium, zinc, chromium, and cadmium.
In other words, compared to these metals, iron is the least corrosion-resistant in natural environments (atmosphere, soil, natural water, etc.). This is due to the following reasons:
The hydrogen and oxygen overpotentials of iron and its oxides are relatively low, making it easy to undergo hydrogen evolution corrosion and oxygen absorption corrosion.
Trivalent iron ions in iron rust and its solutions have good depolarizing effects.
The corrosion products of iron have poor protective properties.
Iron is susceptible to corrosion due to the formation of an oxygen concentration cell.
Iron has weak passivation ability under natural conditions.
Iron forms insoluble corrosion products, commonly known as rust, when corroded in most weakly acidic, neutral, and alkaline solutions. Rust has a porous and loose structure and provides little protection.
In non-oxidizing acids, the corrosion rate increases exponentially with the increase in acid concentration, but in oxidizing acids, the corrosion rate first increases with the increase in acid concentration and then rapidly decreases due to the onset of passivation.
Organic acids are generally weak on iron corrosion, but the corrosion of iron can be accelerated with increasing temperature and oxygen dissolution. Iron is stable in alkaline solutions at room temperature.

The factors affecting the corrosion resistance of carbon steel are:
1. Chemical composition
⑴ The impact of carbon: The carbon content in carbon steel has a significant impact on the corrosion rate of carbon steel in acidic solutions, but the impact is not obvious in neutral solutions.
In non-oxidizing and weak oxidizing media, the corrosion rate of the material increases with the increase of carbon content, because the more carbon content in the steel, the more carbon precipitation in the structure, and the more microbatteries are formed, thus accelerating the corrosion rate.
In oxidative acids, the corrosion rate increases with the increase of carbon content at the beginning, and then decreases when the carbon content reaches a certain level, which is due to the fact that the increase of carbon content is easy to promote the passivation of carbon steel, and the corrosion rate is weakened.
In natural environment and weak acidic water solutions, the impact of carbon content on the corrosion rate of carbon steel is not significant.
This is because oxygen depolarization corrosion is the main factor in such environments, and the performance of protective film on the metal surface and the ease of oxygen reaching the cathode surface in the solution are the main factors, and the carbon precipitation in the steel has little relationship.
⑵ Silicon and manganese generally have almost no obvious impact on the corrosion rate.
⑶ The impact of sulfur and phosphorus
Sulfur is detrimental to the corrosion resistance of steel, and the dissolution rate in acidic solutions increases with the increase of sulfur content.
The increase of sulfur content in steel is easy to cause local corrosion. This is because sulfur is usually present in carbon steel in the form of FeS and MnS, both of which are anodic impurities, causing pitting and sulfide stress corrosion fracture.
Phosphorus in steel is also an active cathode, and harmful in acidic solutions like sulfur. However, phosphorus can effectively improve the corrosion resistance of steel in atmospheric and seawater environments, especially when used with copper, with particularly good results.
⑷ The impact of impurities
For carbon steel, all kinds of impurities will reduce corrosion resistance.
2. Structure impact
The structure of steel depends on its composition and heat treatment state. Generally speaking, the more carbon content in steel, the greater the impact of heat treatment on its corrosion resistance.
When the carbon content is the same, granular pearlite has better corrosion resistance than lamellar pearlite, and the greater the dispersion, the higher the average corrosion rate.
The corrosion resistance of non-passivated carbon steel has a close relationship with its carbon content and heat treatment.
Generally, the higher the carbon content, the worse the corrosion resistance; the corrosion resistance of high-carbon quenched carbon steel is worse, slightly improved after low-temperature tempering, the maximum corrosion rate appears after intermediate-temperature tempering, and after high-temperature tempering, the corrosion rate decreases significantly due to the reduction of active cathode surface area.
Low alloy steel refers to alloy steel with a total amount of alloy elements less than about 5% in carbon steel. According to different purposes, there are many types of alloy elements added to steel, and the amount of these elements also varies greatly, so there are many grades of low alloy steel.
1. Low Alloy Steel Resistant to Atmospheric Corrosion
Low alloy steel resistant to atmospheric corrosion is also known as weathering steel, and is simply referred to as weathering steel.
Its effective alloy elements are copper, phosphorus, and chromium, which enrich the surface of the steel and promote the formation of amorphous states, thereby improving the steel’s resistance to corrosion in atmospheric environments.
Representative low alloy steels resistant to atmospheric corrosion include 16MnCu, 10MnSiCu, 09MnCuPTi, 15MnVCu, 10AuRe, 08MnPRe, etc.
2. Low Alloy Steel Resistant to Seawater Corrosion
In marine environments, the harshest corrosion conditions are in the spray area that is alternately dry and wet, is difficult to protect, and is subject to seawater impact.
The next is the shallow water immersion area.
The effect of alloy elements on the corrosion resistance of steel in different sections is different: copper is the most prominent in improving the corrosion resistance of steel in the spray area, and phosphorus also has a significant effect.
The combination of the two has a better effect. Silicon, molybdenum can reduce the tendency of pitting corrosion of steel in the spray area, chromium, and aluminum also have some effect.
For the corrosion resistance of steel in full immersion conditions, chromium has the most obvious effect, followed by phosphorus, copper, silicon, and nickel.
The low alloy steels resistant to seawater corrosion developed in China mainly include 10MnPNbRe, 09MnCuPTi, 10CrMoAl, 10NiCuAs, 10CrMoCuSi, etc.
3. Low Alloy Steel Resistant to High-temperature and High-pressure Hydrogen and Nitrogen Corrosion
In the petroleum hydrotreating and synthetic ammonia industry, steel works in high-temperature and high-pressure hydrogen environments, and the carbon matrix is easily corroded by interacting with active hydrogen atoms that penetrate into the steel.
Therefore, carbon-alloying elements can be added to the steel, which form stable carbides with carbon, thus improving the hydrogen corrosion resistance of the steel. Studies have shown that the addition of Cr, Mo, and small amounts of V, Nb, and Ti to steel can improve its resistance to hydrogen corrosion.
The low alloy steels resistant to high-temperature and high-pressure hydrogen and nitrogen corrosion in China mainly include 10MoWVNb, 10MoVNbTi, 12SiMoVNb, and 0.8SiWMoTiNb; the typical foreign anti-hydrogen steel 2.25Cr1Mo is currently recognized as one of the best anti-hydrogen steels.
Almost all the hydrotreating reactors in the petrochemical industry are made of this steel.
4. Low Alloy Steel Resistant to Sulfur Corrosion
In the petroleum refining, natural gas, and city gas industries, a large number of low alloy steels are needed to manufacture pipelines, storage tanks, and other equipment, which often work in sulfur-containing environments and are prone to serious sulfur corrosion.
The current research believes that the steel’s microstructure is the key factor affecting the sulfur corrosion fracture of low alloy steels. The formation of martensite microstructure in the steel should be strictly

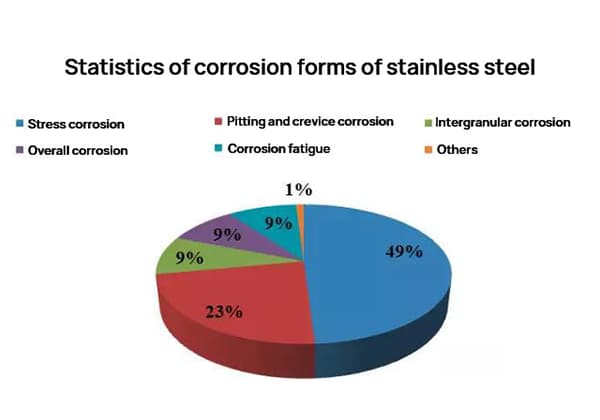
Steel that is resistant to corrosion in atmospheric conditions and neutral electrolytes is known as “stainless steel,” while steel that is resistant to corrosion in chemical reagents and highly corrosive media is known as “acid-resistant stainless steel.”
People generally refer to both stainless steel and acid-resistant stainless steel simply as stainless steel. Stainless steel usually refers to steels with a chrome content greater than 12%, and the term “stainless” is a relative concept. The same steel can be stainless in some environments but not in others.
Classification of stainless steel:
Based on chemical composition, it can be divided into chromium steel, chromium-nickel steel, chromium-manganese steel, etc.
Based on microstructure, it can be divided into martensitic steel, ferritic steel, austenitic steel, and austenitic-ferritic dual-phase steel.
Based on use, it can be divided into seawater-resistant stainless steel, stress-corrosion resistant stainless steel, sulfuric acid-resistant stainless steel, etc.

Chromium stainless steel refers to stainless steel that contains only chromium or is supplemented with a small amount of other alloy elements, excluding Fe and C.
Chromium is the most important alloy element in stainless steel, and has three outstanding roles in improving the corrosion resistance of iron and steel materials:
First, it promotes the passivation of iron-based alloys, improving the passivation ability of the material;
Second, it raises the electrode potential of the solid solution (usually the anode of the corrosion cell), that is, the thermodynamic stability of the matrix structure;
Third, it causes the steel surface to generate a dense and stable surface protective film, thereby improving the corrosion resistance of the steel.
Martensitic stainless steel
Martensitic stainless steel mainly includes Cr13 type (excluding 0Cr13) stainless steel. This type of steel has a high carbon content and can obtain higher strength and hardness through heat treatment, but its corrosion resistance is not as good as ferritic stainless steel and austenitic stainless steel, and the higher the carbon content, the worse the corrosion resistance.
This type of steel is suitable for situations where mechanical properties are required and corrosion resistance is not too high.
Increasing the chromium content of the steel and adding a small amount of nickel can improve the corrosion resistance of martensitic stainless steel; for example, 1Cr17Ni2 is the most corrosion-resistant martensitic, with good resistance to oxidizing acids and most organic acids.
Ferritic stainless steel
Ferritic stainless steel includes Cr13 type, Cr17 type, Cr25-28 type, etc. Due to its high chromium content and low carbon content, its corrosion resistance and high-temperature oxidation resistance are better than martensitic stainless steel, especially its stress corrosion resistance.
However, ferritic stainless steel has poor pitting resistance and intergranular corrosion resistance.
Ferritic stainless steel is mainly used to make equipment and parts that are resistant to high-temperature oxidation, concentrated sulfuric acid corrosion, and gas sulfur corrosion.

Nickel has stronger passive ability than iron, and is also more thermodynamically stable, which is favorable for improving the corrosion resistance of steel.
Especially by adding a certain amount of nickel to stainless steel, a single-phase austenitic stainless steel structure can be obtained, greatly improving the material’s toughness, plasticity and processing performance.
Chrome-nickel stainless steel is the most typical austenitic stainless steel, containing more than 18% of chromium, and more than 8% of nickel, forming types of chrome-nickel stainless steel such as 18-8 (or 18-9), 18-12, 25-20 (HK40), etc.
Chrome-nickel stainless steel has excellent corrosion resistance both in oxidative and non-oxidative media, but its resistance to local corrosion such as stress corrosion, intergranular corrosion, and pitting is poor.
Local corrosion can be inhibited by alloying, such as controlling carbon content, reducing P and N content, and increasing Ni, and adding Si, Mo, Cu, etc. can improve its stress corrosion resistance.
Austenite-ferrite dual-phase steel is another type of chrome-nickel stainless steel, which combines the characteristics of ferrite and austenitic steel and has complementary performance.
In addition, precipitation-hardening (PH) stainless steel also belongs to chrome-nickel stainless steel.
Acid-resistant steel refers to stainless steel with special corrosion resistance in some strong corrosive media.
For certain acid-resistant steel, it only has outstanding corrosion resistance in certain specific media.
Therefore, when selecting acid-resistant steel, it is necessary to comprehensively consider the properties and state of the corrosive medium, and conduct appropriate feasibility tests to ensure that the material can work reliably in strong corrosive media.

Common colored metals used in production include aluminum, copper, magnesium, titanium, and others. In addition, colored metals such as zinc, tin, cadmium, gold, silver, lead are often used as coating materials and linings.
1. Corrosion resistance of pure aluminum
Pure aluminum has poor chemical stability, but has good passivation performance, which can quickly generate dense, well-protected oxide film in the air, and therefore has good corrosion resistance.
Al2O3 is amphoteric, so when the medium pH is less than 4 or greater than 10, the oxide film becomes unstable and damaged, and the protection is lost, causing the corrosion of aluminum to intensify. Aluminum has good corrosion resistance in air and water.
2. Corrosion resistance of aluminum alloys
Aluminum alloys are generally stronger than pure aluminum, but less corrosion-resistant. Aluminum alloys have high corrosion resistance in industrial atmosphere, marine atmosphere, freshwater, and seawater, but may suffer from pitting.
Aluminum alloys have high corrosion resistance in oxidative media due to their ease of passivation, but are easily subject to local corrosion such as pitting, crevice corrosion, stress corrosion in non-oxidative media.
1. Corrosion resistance of magnesium
Magnesium is unstable in most inorganic acids and organic acids, but is quite stable in chromic acid and hydrofluoric acid, which is due to the protective surface film entering the passive state. Magnesium is not corrosion-resistant in marine atmosphere and industrial atmosphere.
2. Corrosion resistance of magnesium alloys
In terms of the corrosion resistance of magnesium alloys, deformable magnesium alloys are less corrosion-resistant than cast magnesium alloys, as they are more sensitive to SCC.
However, in general, the corrosion resistance of magnesium alloys is poor, and effective protective measures need to be taken during use.
1. Copper’s Corrosion Resistance
Copper has a relatively high chemical stability and a positive electrode potential, so it generally does not corrode in acidic solutions.
In non-oxidizing acids, copper has a high degree of chemical stability, but its corrosion resistance is poor in oxidizing acids.
Copper is subject to strong corrosion in other oxidizing media as well.
Copper has good corrosion resistance in various atmospheric conditions, but it is subject to strong corrosion in moist air containing SO2, H2S, and Cl2 gases.
In addition, it is also corroded in ammonium hydroxide and cyanide solutions due to the formation of complex ions.
2. Corrosion Resistance of Copper Alloys
Copper alloys generally have better corrosion resistance than pure copper due to the combined effect of the high thermodynamic stability of the base copper and the protective surface film formed by the alloy elements.
Therefore, the corrosion pattern of copper alloys sometimes also exhibits some characteristics of passive metals.
In non-oxidizing acids, copper alloys have a high degree of chemical stability.
Copper alloys have good corrosion resistance in various atmospheric conditions. Other corrosion resistance is the same as copper.
There are many types of copper alloys, which can be divided into two categories: brass and bronze. Relatively speaking, the corrosion resistance of brass is poor, especially in terms of the tendency to stress corrosion cracking (brass season cracking) and selective corrosion (brass dezincification).
1.Titanium’s Corrosion Resistance
Titanium has poor thermodynamic stability and active chemical properties, but in oxidizing media, a dense protective oxide film is formed on its surface, which is in a stable passive state.
On the one hand, the protective film has good self-healing properties, and on the other hand, it is also very stable in various solutions (including chloride solutions). As a result, titanium has excellent corrosion resistance in many corrosive media and has been widely used in engineering applications.
2. Corrosion Resistance of Titanium Alloys
The corrosion-resistant titanium alloy elements can be divided into two groups: one group is precious metals such as Pd, Ru, Pt, and the addition of trace amounts can significantly improve the corrosion resistance of the alloy.
The other group is Ta, Nb, and Mo, which are cheaper but only have a noticeable anti-corrosion effect when the content is high.
There are not many commercially available titanium alloys with good corrosion resistance. Titanium alloys may experience forms of corrosion such as crevice corrosion, hydrogen brittleness, stress corrosion, welding area corrosion, and natural explosive corrosion during use.
In conclusion, titanium and titanium alloys not only have good corrosion resistance, but also have higher strength and heat resistance than other materials, making them an indispensable structural material for many fields, with a very promising application outlook.
This post mainly introduces the corrosion resistance of some commonly used metals and alloys.
Through the study of this chapter, the focus should be on mastering the corrosion resistance and influencing factors of iron-carbon alloys, stainless steel, and some colored metals, as well as understanding the main functions of corrosion-resistant alloy elements and the scope of application of corrosion-resistant alloys.