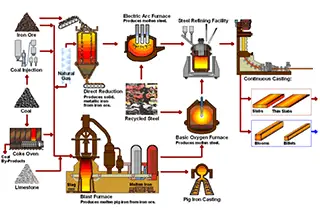
What makes carbon steel and stainless steel different, and why does it matter? In this article, we’ll explore the key distinctions between these two types of steel, focusing on their composition, properties, and uses. You’ll learn how their unique characteristics impact performance in various applications, from construction to culinary tools. Dive in to understand which type of steel is best suited for your specific needs and why choosing the right one is crucial.

Steel
Steel is a collective term for iron alloys with a carbon content ranging from 0.02% to 2.04% by mass. The chemical composition of steel can vary significantly. Steel containing only carbon is called carbon steel or plain steel.
However, in actual production, steel often incorporates different alloying elements based on its intended use, such as manganese, nickel, and vanadium.
Based on performance and usage, they are further divided into structural steel, tool steel, and special performance steel.
Carbon
Present in all steels, carbon is the most vital hardening element. It helps increase the strength of the steel. Tool-grade steel is typically desired to have over 0.6% carbon, also known as high-carbon steel.
Chromium
Chromium enhances wear resistance, hardness, and most importantly, corrosion resistance. If a steel has more than 13% chromium, it is considered to be stainless steel. Nevertheless, all steels can rust if not properly maintained.
Manganese
Manganese is a crucial element that helps in the formation of grain structure, enhancing toughness, strength, and wear resistance. It is used for deoxidizing the steel during heat treatment and rolling processes.
Manganese is present in most types of steel used for knives and scissors, except for A-2, L-6, and CPM 420V.
Molybdenum
As a carbide former, molybdenum prevents steel from becoming brittle and maintains steel strength at high temperatures. It is found in many steels.
Air-hardening steels (like A-2, ATS-34) always contain 1% or more molybdenum, enabling them to harden in air.
Nickel
Nickel maintains strength, corrosion resistance, and toughness. It is present in L-6, AUS-6, and AUS-8.
Silicon
Silicon helps increase strength. Like manganese, it is used in the steel production process to maintain steel strength.
Tungsten
Tungsten enhances wear resistance. It is mixed with an appropriate ratio of chromium or manganese to produce high-speed steel. High-speed steel M-2 contains a significant amount of tungsten.
Vanadium
Vanadium enhances wear resistance and ductility. A carbide of vanadium is used in the manufacture of steel bars. Many types of steel contain vanadium, including M-2, Vascowear, CPM T440V, and 420VA, which have a high vanadium content.
The main difference between BG-42 and ATS-34 is the former’s vanadium content.
a. Carbon structural steel:
(a) Q195;
(b) Q215 (A, B);
(c) Q235 (A, B, C);
(d) Q255 (A, B);
(e) Q275.
b. Low-alloy structural steel
c. Common structural steel for specific purposes
a. Structural steel:
(a) High-quality carbon structural steel;
(b) Alloy structural steel;
(c) Spring steel;
(d) Free-cutting steel;
(e) Bearing steel;
(f) High-quality structural steel for specific purposes.
b. Tool steel:
(a) Carbon tool steel;
(b) Alloy tool steel;
(c) High-speed tool steel.
c. Special performance steel:
(a) Stainless acid-resistant steel;
(b) Heat-resistant steel;
(c) Electric heating alloy steel;
(d) Electrical steel;
(e) High manganese wear-resistant steel.

The main mechanical properties of steel depend on its carbon content. Steel that does not contain a large amount of alloying elements is sometimes referred to as plain carbon steel or carbon steel.
Carbon steel, also known as plain carbon steel, refers to iron-carbon alloys with a carbon content (WC) of less than 2%.
In addition to carbon, carbon steel generally contains small amounts of silicon, manganese, sulfur, and phosphorus.
Carbon steel can be classified into three types based on its application: carbon structural steel, carbon tool steel, and free-cutting structural steel. Carbon structural steel can be further divided into structural steel for buildings and structural steel for machinery.
According to the smelting method, carbon steel can be divided into open-hearth steel, converter steel, and electric furnace steel.
According to the deoxidation method, carbon steel can be classified as boiling steel (F), killed steel (Z), semi-killed steel (b), and special killed steel (TZ).
Based on carbon content, carbon steel can be categorized into low-carbon steel (WC ≤ 0.25%), medium-carbon steel (WC 0.25%-0.6%), and high-carbon steel (WC > 0.6%).
Based on phosphorus and sulfur content, carbon steel can be divided into ordinary carbon steel (higher phosphorus and sulfur content), high-quality carbon steel (lower phosphorus and sulfur content), superior high-quality steel (even lower phosphorus and sulfur content), and special high-quality steel.
Generally, as the carbon content increases in carbon steel, the hardness and strength also increase, but the ductility decreases.

Stainless steel, also known as acid-resistant steel, is composed of two major components: stainless steel and acid-resistant steel. In simple terms, steel that can resist atmospheric corrosion is called stainless steel, while steel that can withstand chemical media corrosion is called acid-resistant steel. Stainless steel is a high-alloy steel with over 60% iron as its base, incorporating alloying elements such as chromium, nickel, and molybdenum.
When the steel contains more than 12% chromium, it is resistant to corrosion and rust in the atmosphere and dilute nitric acid. This is because chromium can form a tightly adhering chromium oxide film on the steel surface, effectively protecting it from corrosion. The chromium content in stainless steel is generally over 14%, but stainless steel is not entirely immune to rusting.
In coastal areas or places with severe air pollution, when the air contains a large amount of chloride ions, the exposed surface of stainless steel may develop some rust spots. However, these rust spots are limited to the surface and do not corrode the internal matrix of stainless steel.
Generally, steel with a chromium content (Wcr) greater than 12% exhibits the characteristics of stainless steel. Stainless steel can be further classified into five categories based on its microstructure after heat treatment: ferritic stainless steel, martensitic stainless steel, austenitic stainless steel, austenitic-ferritic (duplex) stainless steel, and precipitation-hardening stainless steel.
Ferritic Stainless Steel: Contains 12% to 30% chromium. Its corrosion resistance, toughness, and weldability improve with increasing chromium content. It exhibits better resistance to chloride stress corrosion cracking than other types of stainless steel.
Austenitic Stainless Steel: Contains over 18% chromium, along with approximately 8% nickel and small amounts of molybdenum, titanium, nitrogen, and other elements. It has excellent comprehensive properties and can resist corrosion in various media.
Austenitic-Ferritic (Duplex) Stainless Steel: Combines the advantages of austenitic and ferritic stainless steel, and exhibits superplasticity.
Martensitic Stainless Steel: Has high strength but poor ductility and weldability.

Color: Stainless steel contains more chromium and nickel, resulting in a silver-like appearance. Carbon steel mainly consists of carbon and iron, with fewer other metal elements, giving it a predominantly iron color that is darker.
Surface Texture: Stainless steel, with its higher content of other metal elements, has a smooth surface. Carbon steel, containing more iron and carbon, has a rougher surface and lacks the smoothness of stainless steel.
Magnetism: Carbon steel has magnetic properties on its surface and can be attracted by a magnet. Stainless steel is generally non-magnetic under normal conditions and is not attracted to magnets.
Carbon Content: Carbon steel’s mechanical properties depend on its carbon content, with steel containing less than 2% carbon, and generally not adding a significant amount of alloying elements. In contrast, stainless steel, in order to maintain its corrosion resistance, has relatively low carbon content, typically not exceeding 1.2%.
Alloy Content: Carbon steel contains a small amount of alloying elements, such as silicon, manganese, sulfur, and phosphorus. Stainless steel has a higher content of alloying elements, primarily chromium and nickel, exceeding 12%.
Corrosion Resistance: Carbon steel, with its low alloy content, exhibits weaker corrosion resistance. Stainless steel, with its higher content of chromium and nickel, possesses stronger corrosion resistance.
The distinction between carbon steel and stainless steel primarily lies in their corrosion resistance. However, stainless steel, with its superior properties, serves functions that other types of steel can’t replace in practical applications.
For instance, some heat-resistant stainless steels and stainless steels with excellent surface characteristics are widely used as decorative materials.
Additionally, stainless steel’s exceptional mechanical properties make it indispensable in various manufacturing sectors.
Ordinary steel, also known as carbon steel, is an iron-carbon alloy. It is categorized into low carbon steel, medium carbon steel, and cast iron based on the carbon content.
Generally, steel with less than 0.2% carbon is called low carbon steel, also known as soft iron or pure iron; steel with a carbon content between 0.2-1.7% is called steel; and steel with more than 1.7% carbon is called pig iron.
1. Steel with a chromium content higher than 12.5% possesses a high resistance to corrosion from external media (acid, alkali salt) and is therefore referred to as stainless steel.
Depending on the internal structure of the steel, stainless steel can be divided into martensitic, ferritic, austenitic, ferritic-austenitic, and precipitation-hardened types, with a total of 55 types specified by national standard GB3280-92.
In everyday life, we frequently encounter austenitic stainless steel (some call it nickel stainless) and martensitic stainless steel (some refer to it as “stainless iron,” which is scientifically incorrect and prone to misunderstanding).
Typical grades of austenitic stainless steel include 0Cr18Ni9, or “304,” and 1Cr18Ni9Ti. Martensitic stainless steel, used for manufacturing scissors and knives, primarily includes grades 2Cr13, 3Cr13, 6Cr13, 7Cr17, etc.
2. Differences in the compositions of these two types of stainless steel result in different internal metal microstructures.
3. Austenitic stainless steel, due to the high chromium and nickel content (approximately 18% chromium and more than 4% nickel), exhibits an austenitic internal structure.
This structure is non-magnetic and cannot be attracted by a magnet. It is commonly used for decorative materials, such as stainless steel pipes, towel racks, cutlery, stoves, etc.
4. Martensitic stainless steel is used to manufacture knives and scissors. Since cutting tools must be sharp, they must possess a certain hardness.
This type of stainless steel must undergo heat treatment to change its internal structure and increase its hardness to be used as a cutting tool.
But this type of stainless steel has a tempered martensitic internal structure and is magnetic, meaning it can be attracted by a magnet.
Therefore, one cannot simply determine whether a material is stainless steel based on its magnetism.
The distinction between stainless steel seamless pipes and carbon steel seamless pipes primarily lies in the different design rules for these two types of steel, meaning their design rules aren’t interchangeable. The differences can be summarized as follows:
Firstly, stainless steel hardens during cold working due to a phenomenon called work hardening. For instance, during bending, it exhibits anisotropy, with different properties in the transverse and longitudinal directions.
The strength increase from cold working can be utilized to enhance the safety factor, particularly when the bent area is small compared to the total area, making the increase negligible.
Secondly, the stress-strain curve for stainless steel differs from that of carbon steel. The elastic limit of stainless steel is approximately 50% of its yield stress, which, as per standard regulations, is lower than the yield stress of medium carbon steel.
Lastly, stainless steel doesn’t have a defined yield point. Instead, the yield stress is generally represented by σ0.2 and is considered an equivalent value.
Heat treatment is a process that manipulates the physical properties of a metal by utilizing heating and cooling. Through heat treatment, the microstructure of steel can be improved to meet specific physical requirements.
Some of the characteristics achieved through this process include toughness, hardness, and wear resistance. These properties are obtained using heat treatment techniques such as hardening, tempering, annealing, and surface hardening.
Hardening, also known as quenching, involves uniformly heating the metal to an appropriate temperature, then rapidly immersing it in water or oil for abrupt cooling, or cooling it in air or a freezing area to achieve the desired hardness.
Tempering is necessary after hardening, as the steel becomes brittle and susceptible to fracture due to the stress induced by rapid cooling.
To eliminate this brittleness, tempering is performed by reheating the steel to an appropriate temperature or color, followed by rapid cooling.
Although this process slightly reduces the hardness of the steel, it increases its toughness and reduces its brittleness.
Annealing is a method used to eliminate the internal stress in steel and to homogenize it. The process involves heating the steel above its critical temperature and then placing it in dry ash, lime, asbestos, or sealing it inside a furnace to allow it to cool slowly.
Hardness refers to the ability of a material to resist penetration by an external object. A common method of testing steel hardness is by using a file on the edge of the workpiece, where the depth of the filing marks indicates the degree of hardness.
However, this method is not highly accurate. Modern hardness testing is typically done with a hardness tester. The Rockwell hardness test is one of the most commonly used tests.
The Rockwell hardness tester measures the depth of a diamond indenter penetration into the metal; the deeper the penetration, the lower the hardness. The depth of penetration can be read accurately from a dial, and this reading is referred to as the Rockwell hardness number.
Forging is a process where metal is shaped by hammering. When steel is heated to forging temperature, it can be forged, bent, drawn, and shaped. Most steel is easy to forge when heated to a bright cherry-red color. One common method to increase the hardness of steel is through quenching.
Brittleness refers to the tendency of a metal to fracture easily. Cast iron, for example, is highly brittle and can even crack when dropped. There is a close relationship between brittleness and hardness; typically, materials with high hardness also have high brittleness.
Ductility (also known as malleability) refers to the ability of a metal to permanently deform without fracturing when subjected to external forces. Ductile metals can be drawn into thin wires.
Elasticity refers to the property of a metal to deform under external forces and to return to its original shape once the forces are removed. Spring steel is a highly elastic material.
Malleability also known as forgeability, is another description of the ductility or softness of a metal. Malleability is the property of a metal to deform without fracturing when subjected to hammering or rolling.
Toughness is the ability of a metal to withstand vibrations or impacts. Toughness is the opposite of brittleness.