What Is Welding Flux?
Welding flux is a granular welding material that, when melted during welding, forms slag and gas. This substance plays a crucial role in protecting the molten metal and aiding in metallurgical treatment.
Flux is usually a mixture mainly composed of rosin, an auxiliary material that ensures the smooth progress of the welding process. Welding is a major process in electronic assembly, and flux is an auxiliary material used during welding.
The main function of the flux is to remove oxides from the solder and the surface of the base material being soldered, achieving the necessary surface cleanliness.
It prevents re-oxidation of the surface during welding, reduces surface tension of the solder, and improves welding performance. The quality of the flux directly affects the quality of electronic products.
Flux Composition
The welding flux is composed of a mixture of minerals, including marble, quartz, fluorite, and others, as well as chemicals such as titanium dioxide and cellulose.
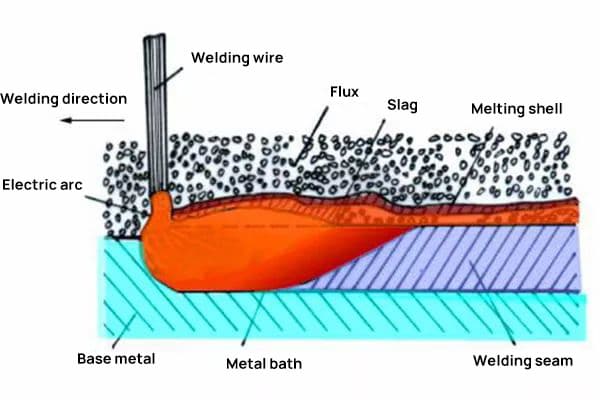
The primary application of welding flux is in submerged arc welding and electroslag welding.
Related reading: Types of welding
In the past few decades, in the soldering process of electronic product production, rosin resin flux composed mainly of rosin, resin, halide-containing activators, additives, and organic solvents are often used.
Although this type of flux has good solderability and low cost, it has high post-weld residues. These residues contain halide ions, which gradually cause problems such as decreased electrical insulation performance and short-circuits.
To solve this problem, it is necessary to clean the rosin resin flux residues on the electronic printed board, which not only increases production costs, but also the solvent used to clean the rosin resin flux residues is mainly fluorochloro compounds. This compound is a substance that depletes the atmospheric ozone layer and is currently banned and phased out.
However, for various reasons, many companies still use the process of soldering with rosin resin flux and then cleaning with a fluorochlorine cleaner, which has low efficiency and high cost, and causes serious environmental pollution.
The no-clean flux, which is used more in the market and is of a higher grade, is composed of: organic solvents, natural resins and their derivatives, synthetic resin surfactants, organic acid activators, anti-corrosive agents, co-solvents, and film-forming agents.
Simply put, it is a homogeneous transparent mixed solution formed by dissolving various solid components in various liquids, where each component has different proportions and functions.
Organic solvents:
A mixture of one or several types of ketones, alcohols, esters, commonly used ones include ethanol, propanol, butanol; acetone, toluene isobutyl ketone; ethyl acetate, butyl acetate, etc.
As a liquid component, its main function is to dissolve the solid components in the flux to form a homogeneous solution, making it easier for the components to be soldered to evenly coat an appropriate amount of flux components, while it can also clean light dirt and oil on the metal surface.
| Constituent Components | Primary Function | |
| Volatile Components | Solvent | Regulation of viscosity and dispersion of solid components |
| Solid Composition | Resin | Primary ingredients, catalytic soldering functions |
| Dispersant | Prevention of separation, fluidity characteristics | |
| Activator | Deoxidation | |
Natural Resin and its Derivatives or Synthetic Resin Surfactants:
Halogen-containing surfactants have high activity and soldering ability, but because halogen ions are difficult to clean, ion residues are high, and halogen elements (mainly chlorides) have strong corrosive properties, they are not suitable for use as raw materials for no-clean flux.
Non-halogen-containing surfactants have slightly weaker activity, but fewer ion residues. Surfactants are mainly non-ionic surfactants of the fatty acid family or aromatic family. Their main function is to reduce the surface tension generated when the solder contacts the metal of the lead, enhance the surface wetting force, enhance the penetration of organic acid activators, and also play a role as a foaming agent.
Organic Acid Activator:
Composed of one or several kinds of dicarboxylic acids or aromatic acids, such as succinic acid, glutaric acid, itaconic acid, salicylic acid, fumaric acid, heptanoic acid, malic acid, succinic acid, etc., its main function is to remove oxides on the lead foot and the surface of the molten solder, and it is one of the key components of the flux.
Anti-corrosive Agent:
Reduces the residues of solid components such as resins and activators after high-temperature decomposition.
Co-solvent:
Prevents the tendency of solid components such as activators to desorb from the solution, avoiding the poor uniform distribution of activators.
Film-forming Agent:
During the soldering process of the lead, the applied flux precipitates and crystallizes to form a uniform film. The residues after high-temperature decomposition can be quickly solidified, hardened, and reduced in stickiness due to the presence of the film-forming agent.

Working Principle of Flux
The working principle of flux is easy to understand. In a nutshell: during the entire welding process, the flux removes the oxide layer on the surface of the welding material through the action of its own active substances.
At the same time, it reduces the surface tension between the tin liquid and the welding material, enhancing the flow and wetting properties of the tin liquid, thus helping complete the welding. Hence the name “flux”.
To fully analyze the working principle of flux, it involves using the activators in the flux to clean the oxides on the surface of the welding material, enabling the solder alloy to bond well with the welding material and form a weld point. The substances that play a major role in this process are the activators in the flux, which can quickly remove the oxides of the solder pads and component pins, and sometimes also protect the welding material from further oxidation before the welding is completed.
In addition, while removing the oxide film, the surfactants in the flux also start to work. They can significantly reduce the surface tension of the liquid solder on the surface of the welding material, enhance the fluidity and spreading ability of the liquid solder, and ensure that the tin solder can penetrate every tiny brazing gap.
In the tin furnace welding process, the moment the welded body leaves the surface of the tin liquid, due to the wetting action of the flux, the excess tin solder will flow down along the pin, thereby avoiding poor phenomena such as solder spikes and bridging.
Functions of Welding Flux
Functions of the flux:
(1) Remove oxides from the welding surface, reduce the melting point and surface tension of the solder, and reach the brazing temperature as quickly as possible.
(2) Protect the liquid-state weld metal from being affected by harmful gases in the surrounding atmosphere.
(3) Enable the liquid solder to have an appropriate flow rate to fill the brazing seam.
(4) Destroy the metal oxide film to clean the solder surface, which is conducive to solder wetting and the generation of solder joint alloys.
(5) Can cover the surface of the solder, preventing the solder or metal from continuing to oxidize.
(6) Enhance the activity of the solder and the surface of the metal being soldered, reducing the surface tension of the solder.
(7) The solder and flux are fused, which can increase the flowability of the solder and further improve the wettability.
(8) It can accelerate the transfer of heat from the soldering iron head to the surface of the solder and the soldered object.
(9) An appropriate flux can also make the solder joint look better.
Functions of the flux in submerged arc welding:
(1) . Mechanical protection: Under the action of the electric arc, the flux melts into the surface slag, protecting the liquid-state weld metal from the intrusion of gases in the surrounding atmosphere, thereby preventing gas inclusions in the weld seam.
(2) . Transfer necessary metal elements to the molten pool.
(3) . Promote a smooth and straight weld seam surface with good formation. The melting point of the flux should be 10-30℃ lower than the solder melting point, under special circumstances, the melting point of the flux can be higher than the solder.
If the melting point of the flux is too low compared to the solder, the flux will melt prematurely, causing the flux composition to lose activity when the solder melts due to evaporation and interaction with the parent material.
The choice of flux usually depends on the nature of the oxide film. Alkaline oxide films, such as Fe, Ni, Cu, etc., often use acidic fluxes containing boron anhydride (B2O3), acidic oxide films, for example, to deal with cast iron containing high SiO2 oxide film, often use alkaline Na2CO3 flux to form easily melted Na2SiO3 and enter the slag. Some fluoride gases are also often used as fluxes, they react uniformly and leave no residue after welding.
BF3 is often mixed with N2 for brazing stainless steel at high temperatures. Fluxes used for brazing below 450℃ are soft fluxes, which are divided into two types, one is water-soluble, usually composed of single or mixed salt solutions of hydrochloric acid and phosphoric acid, they have high activity and strong corrosiveness, and need to be cleaned after welding.
The other is a water-insoluble organic flux, usually based on rosin or synthetic resin, with added organic acid, organic amine, or its HCl or HBr salt to enhance defilming capability and activity.
Conditions that Commonly Used Flux
(1) The melting point should be lower than the solder.
(2) The surface tension, viscosity, and density should be less than that of the solder.
(3) It should not corrode the parent material, and it should increase the flowability of the solder and remove the oxide film on the metal surface at the welding temperature.
(4) The flux residue is easy to remove.
(5) It should not produce toxic gas and odor to prevent harm to the human body and environmental pollution.
Types of Welding Flux
There are several ways to classify welding flux, including its usage, method of manufacture, chemical composition, metallurgical properties during welding, and the pH and particle size of the flux.
Regardless of the classification method used, it only highlights certain aspects of the welding flux and does not fully encompass all its characteristics.
Common classification methods include:
1. Neutral welding flux
A neutral welding flux is one that does not significantly alter the chemical composition of the deposited metal or the welding wire after welding.
This type of flux is often used for multi-pass welding, particularly when the base metal has a thickness greater than 25 mm.
The characteristics of a neutral welding flux are as follows:
a. The flux contains little to no oxides, such as SiO2, MnO, and FeO.
b. The flux does not cause oxidation in the weld metal.
c. Welding a seriously oxidized base metal may result in porosity and cracks in the weld bead.

2. Active welding flux
An active welding flux is one that contains a small amount of deoxidizing agents, such as Mn and Si. This type of flux can improve resistance to porosity and cracking.
The following are the characteristics of an active welding flux:
a. The presence of deoxidizers such as Mn and Si can cause changes in the chemical composition of the deposited metal as the arc voltage fluctuates. An increase in Mn and Si can increase the strength of the deposited metal but decrease its impact toughness. Hence, it is important to closely control the arc voltage during multi-pass welding.
b. The active welding flux has a strong ability to prevent porosity.

3. Alloy welding flux
An alloy welding flux contains additional alloy components that serve as transition elements. Most alloy welding fluxes are sintered.
This type of flux is primarily used for welding low-alloy steel and for wear-resistant surfacing.
4. Melting welding flux
Melting welding flux is produced by combining raw materials of various minerals in a specific proportion, heating them to over 1300 ℃, melting and thoroughly mixing them, and then cooling them in water to form granules.
The process continues with drying, crushing, sieving, and packaging for use.
In China, a common brand of melting welding flux is “HJ”. The first digit after the “HJ” designation indicates the content of MnO, the second digit represents the content of SiO2 and CaF2, and the third digit distinguishes between different brands of the same type of welding flux.
5. Sintered welding flux

After proportioning the ingredients, dry mixing is performed and a binder (water glass) is added for wet mixing. The mixture is then granulated.
Next, it is sent to a drying oven for curing and drying, and finally sintered at approximately 500 degrees.
In China, a common brand of sintered welding flux is represented by “SJ”. The first digit after the “SJ” designation indicates the slag system, while the second and third digits distinguish between different brands of the same type of slag system flux.
6. Other Classification Methods
The types of flux can be broadly divided into organic, inorganic, and resin series.
Resin flux is usually extracted from the secretions of trees. It is a natural product with little corrosiveness. Rosin is a representative of this type of flux, so it is also called rosin flux.
As the flux is usually used in combination with solder, it can be divided into soft flux and hard flux corresponding to the solder.
Commonly used in the assembly and maintenance of electronic products are rosin, rosin mixed flux, solder paste and hydrochloric acid and other soft flux. They should be selected according to different welding workpieces in different occasions.
Control of Welding Flux
- Control of flux drying and heat preservation
Before using the flux, it should first be baked according to the instructions of the flux. This drying standard is obtained through tests and process inspection control and is a quality-assured, correct data. It is an enterprise standard, and different enterprises have different requirements.
Next, refer to the flux drying temperature and retention time recommended by JB4709-2000 “Welding Procedure for Steel Pressure Vessels”. Generally, when drying flux, the pile height does not exceed 5cm. The welding material library often dries more rather than less at one time, and prefers thickness over thinness in stacking. Strict management should be enforced in this regard to ensure the quality of flux drying.
Avoid excessive stacking thickness and ensure thorough drying of the flux by extending the drying time.
- Control of flux on-site management and recycling disposal
The welding area should be cleaned, and debris must not be mixed into the flux, including the flux used for flux padding should be issued according to regulations, preferably kept at around 50℃, timely recycling of the flux to avoid contamination. The flux used repeatedly uses 8 and 40 mesh sieves to sieve and remove impurities and fine powder, and then mixed with three times the new flux for use.
Before use, it must be dried at 250-350℃ and kept warm for 2 hours. After drying, it is kept in a 100-150℃ incubator for the next use, and outdoor storage is prohibited. In complex field conditions or high relative humidity, timely management of the site should be done, keep it clean, conduct necessary tests for flux moisture resistance and mechanical mixtures, control moisture absorption rate and mechanical inclusions, avoid disorderly stacking, and flux mixing.
- Flux particle size and distribution requirements
The flux has certain particle size requirements, the particle size should be appropriate, so that the flux has a certain permeability, the welding process does not emit continuous arc light, and avoids air pollution to the molten pool to form pores. Fluxes are generally divided into two types, one with a common particle size of 2.5-0.45mm (8-40 mesh), and the other with a fine particle size of 1.43-0.28mm (10-60 mesh).
Fine powder smaller than the specified particle size is generally not more than 5%, and coarse powder larger than the specified particle size is generally more than 2%. Tests and control of the particle size distribution of the flux should be carried out to determine the welding current used.
- Control of flux particle size and heap scatter height
If the flux layer is too thin or too thick, it will cause pits, spots, and pores on the surface of the weld, forming an uneven weld path shape. The thickness of the flux layer should be strictly controlled within the range of 25-40mm. When using sintered flux, due to its low density, the pile height of the flux is 20%-50% higher than that of fused flux. The larger the diameter of the welding wire, the higher the welding current, and the higher the flux layer thickness accordingly.
Due to the non-standard operation of the welding process and the unfair treatment of fine powder flux, there will be intermittent uneven pits on the surface of the weld, the non-destructive testing is qualified but the appearance quality is affected, and the shell thickness is locally weakened.
How to Choose the Right Flux?
For the users, it is impossible to test the composition of the flux. If you want to know whether the flux solvent evaporates, you can simply measure it from the specific gravity. If the specific gravity increases a lot, you can determine that the solvent has evaporated.
When choosing flux, there are a few suggestions for the users:
(1) Smell the odor to preliminarily determine what kind of solvent is used. The smell of methanol is relatively small but pungent, the smell of isopropanol is a bit heavier, and ethanol has a fragrant smell.
Although the supplier may also use a mixed solvent, the supplier is usually willing to provide a composition report upon request.
However, the price of isopropanol is about 3 to 4 times that of methanol, so if you press the supplier for a lower price, the quality may be questionable.
(2) Determine the sample, this is also the most fundamental method for many companies to choose flux. When confirming the sample, the supplier should be asked to provide the relevant parameter report and compare it with the sample.
If the sample is confirmed to be OK, the subsequent delivery should be compared with the original parameters. If any abnormality appears, check the specific gravity, acidity value, etc.
(3) The current flux market is mixed. When choosing, you should have a clear understanding of the supplier’s qualifications.
FAQs About Welding Flux
- What is a flux in welding?
Flux in welding is a material used to promote, facilitate, and protect the fusion of metals during the welding process. It is used to prevent the formation of oxides and other unwanted byproducts that can form due to heat. Flux can be in the form of a liquid, paste, or solid material, and it helps in creating a better, cleaner, and stronger weld.
- Do you need flux for stick welding?
Yes, flux is essential in stick welding. The electrode used in stick welding, often referred to as a “stick,” is covered with flux. As the stick is used up, the flux creates a gas shield around the weld area, protecting the molten metal from the surrounding air, which can lead to contamination and weak welds if not protected.
- Is flux welding as strong as MIG?
The strength of a weld is primarily determined by the skill of the welder and the preparation of the materials, rather than the type of welding. That said, MIG (Metal Inert Gas) welding tends to produce cleaner and sometimes stronger welds than flux core welding because of its use of a shielding gas. However, flux welding is more versatile and works better on thicker, dirtier, and rustier materials.
- What is flux welding best for?
Flux welding is best used in situations where it is difficult to control the environmental conditions, such as outdoors or in drafty conditions. This is because the flux creates a protective barrier that shields the weld from atmospheric gases. It’s also great for welding thicker, rougher materials and when welding out of position. In addition, it’s usually more economical and easier to learn than other types of welding.