
Abstract:
Non-metallic inclusions play a crucial role in determining many properties of steel and have a significant impact on the processing and utilization of steel products.
This article provides an overview of the latest developments in the origin and classification of non-metallic inclusions and summarizes the research work done in recent decades.
It highlights the dynamic conditions of inclusion formation and the effect of current smelting conditions on the composition, quantity, and size distribution of inclusions.
The study of inclusion denaturation focuses on steel types such as radial steel wire, spring steel, and bearing steel to achieve the desired size and shape of inclusions.
Additionally, measures must be taken to prevent clogging of the continuous casting nozzle by flocculent flow.
With the advancement of micro electron microscopy, the distribution characteristics of inclusions are now more clearly understood. The exciting field of “oxide metallurgy” has also been covered in the context of inclusion engineering.
Finally, the article touches upon the improvement of inclusion characteristics and the challenges of conducting a quantitative analysis.
In recent decades, remarkable progress has been made in controlling inclusions in steel, largely due to a deeper understanding of the interplay between thermodynamics, slag composition of molten steel, and the steelmaking process. This enables the optimization of inclusions and processes to enhance the properties of steel.
However, there are still some important challenges that need to be addressed, and the process of inclusion control and optimization must be continuously improved.
It was not until fifty years ago that people started paying attention to the study of non-metallic inclusions (NMIs) in steel. At that time, it was believed that these inclusions were the result of the corrosion of refractory materials and the presence of various mold fluxes and top slags.
However, despite its importance, the study of inclusions was not as widely recognized as it is today. This was because physical metallurgists generally focused on the study of metallic phases, rather than non-metallic phases like inclusions in steel.
As the demand for high-performance steel with severe service conditions increased, the correlation between the type, size, and distribution of NMIs and the performance of steel became more apparent. This led to a growing interest in studying the origin, characteristics, and behavior of inclusions in the smelting and processing of steel products.
Since the 1980s, significant progress has been made in controlling and quantitatively analyzing NMIs, and the impact of these inclusions on the properties of steel has been widely researched. Inclusion control engineering has become a crucial aspect of smelting, with the aim of achieving the desired inclusion characteristics through appropriate process design and production of steel.
In this article, we cover the origin and control of NMIs, the behavior of inclusions during machining, the quantitative analysis and distribution characteristics of inclusions, and the latest developments in inclusion control engineering. However, we do not delve into the influence of inclusions on the properties of steel in detail, as this is an extensive and rapidly growing field.
For those interested in exploring this topic further, classic books by Kiessling and the conferences and papers of the International Clean Steel Organization, held every 3-5 years and sponsored by the Hungary Mining and Metallurgical Association, are valuable sources of knowledge.
With the ongoing advancements in modern steelmaking technology, it is observed that oxidation reactions and refining methods are employed to eliminate detrimental elements from steel.
These impurities, such as sulfur from coal and coke, can penetrate liquid iron and steel, but their solubility in solid solution steel is quite limited.
During solidification, the molten steel moves from the crystallization front to liquid steel, eventually forming low melting point compounds such as “FeO” and “FeS” or eutectic that contains both compounds. As a result, this steel is unsuitable for hot working processes such as rolling and forging.
The oxides, sulfides, and alloy elements (such as Mn) in steel exhibit a complex relationship. However, to produce high-quality steel, the content of oxygen and sulfur dissolved in the molten steel must be reduced.
Elements like Mn, Al, Si can be utilized as alloy elements in steel, as they possess high affinity with oxygen and can be deoxidized in the molten steel. These deoxidized elements become oxide non-metallic inclusions.
On the other hand, the steel contains sulfur, and the solubility of Ca and Mg in the steel is minimal. Their affinity with rare earth and S is high enough to form non-metallic sulfide inclusions with a low melting point.
As a result, most of the sulfur in the steel is eliminated via refining and enters the slag, while the remaining sulfur precipitates sulfide inclusions during solidification.
These non-metallic inclusions can be classified into two categories based on their type: the chemical composition of the inclusions (such as oxide and sulfide inclusions) and the stage of inclusion formation.
Solidification marks the boundary point in the formation stage of inclusions. The inclusions formed prior to solidification are known as primary inclusions, while those formed during and after solidification are referred to as secondary inclusions.
In addition to these classifications, other commonly used categorizations can also be confusing, such as the source of inclusions. Inclusions formed during the steelmaking process (such as oxide and sulfide inclusions) are classified as “endogenous” inclusions, while those originating from outside sources (such as refractory chips and mold powder) are referred to as “exogenous” inclusions.
In general, there are only a few large particle exogenous inclusions that remain independent from the molten steel for an extended period of time and do not react with it. This has changed from the past, when it was believed that such large particle inclusions came from the refractory and casting mold. However, in modern steel, these large particle inclusions have been significantly reduced.
Some students may mistakenly believe that exogenous inclusions are the most important non-metallic inclusions, but this does not mean that the interaction between the molten steel and the refractory is insignificant. The presence of these inclusions in steel indicates that the molten steel and refractory do have an impact. If the definition of exogenous inclusions is expanded to include secondary oxidation and the involvement of mold powder, it provides a more appropriate definition of exogenous inclusions.
However, there is still debate about the classification of these inclusions, as they can change during the smelting process and it is not always clear which process is involved.
Finally, a common method of classifying non-metallic inclusions is based on their size, dividing them into macro inclusions and micro inclusions. The classification proposed by Kiessling is often used, where inclusions that cause instant damage to the steel products during processing or use are considered macro inclusions.
It should be noted that the size classification of inclusions is subjective, and randomly dividing inclusions into macro and micro based on size is difficult to define.
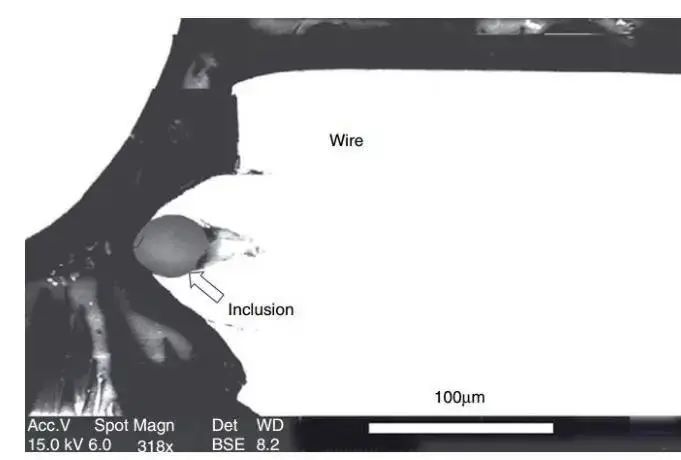
Fig. 1 Fracture of tire radial wire during drawing due to the existence of large particle hard phase.
The inclusion is indicated by an arrow, and the photo was taken using a scanning electron microscope backscatter technique.
The high temperature during steelmaking brings the inclusion formation reaction close to the state of equilibrium. As a result, thermodynamics has become a crucial tool for understanding inclusions.
For a long time, the thermodynamic basis of inclusion formation has been a focus of research. However, the thermodynamic formation data related to inclusions in aluminum oxide remains inconsistent and contradictory, making it an area worth further study.
Aluminum is commonly used as a deoxidizer in steelmaking, and the thermodynamic calculation of inclusions generated during the process is not problematic. However, the smelting process of new-generation steel materials with high aluminum and high manganese content introduces uncertainty.
The situation becomes more complex when the dissolution of Mg and Ca in steel is extremely limited. Despite this, the related thermodynamic data of these elements is still widely studied in the steelmaking process.
In recent decades, thermodynamic calculation has been applied to solve complex problems in iron and steel production and smelting. However, conventional calculation methods still struggle to address these issues.
Many literatures have discussed the application of thermodynamic calculation in various aspects of iron and steel production and smelting, as well as related inclusion problems.
Classifying inclusions into primary and secondary can be useful in discussing their formation and removal. In principle, it is possible to eliminate primary inclusions in steel.
However, secondary inclusions form during solidification and cannot be removed. The best that can be done is to modify them to minimize their negative impact on steel.
3.1.1 Nucleation and structure of inclusions
From the perspective that the primary inclusions formed in liquid steel are closely tied to the thermodynamic process, two key aspects are taken into consideration: their nucleation and the structures that result from it.
In general, when a deoxidizer is added to molten steel, it will nucleate quickly. This is because high supersaturation is observed during the addition and dissolution of the deoxidizer. Sigworth and Elliott conducted a thorough evaluation of silicon nucleation conditions and found that supersaturated dissolved oxygen is a requirement.
However, Miyashita’s research and industrial observations did not demonstrate clear supersaturation during silicon deoxidation in steelmaking. Miyashita also compared dissolved oxygen and total oxygen and found that the rate of total oxygen reduction is determined by the removal rate of deoxidized products, as illustrated in Figure 2.

Fig. 2 Total oxygen and dissolved oxygen in steel after silicon deoxidation in the molten bath are measured as a function of time.
In many studies on deoxidation, the difference between the total oxygen content and the dissolved oxygen content in steel is dependent on the quantity of oxide inclusions that are generated. This key conclusion is demonstrated in Figure 3.
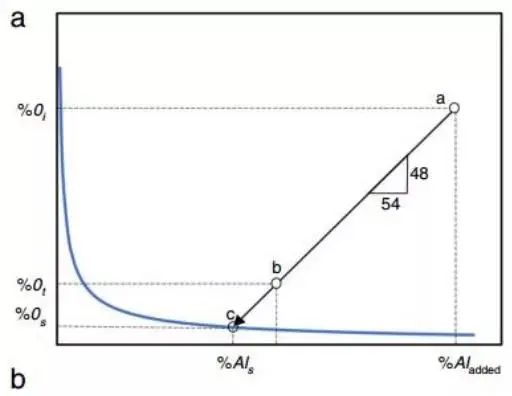

Fig. 3
This is a basic illustration of how the total oxygen and dissolved oxygen in steel are affected by the amount of oxide inclusions.
In the example, deoxidation begins at point “a” and aluminum is added to the steel, starting with a certain percentage of dissolved oxygen, Oi.
Without nucleation conditions at the nucleation boundary, aluminum oxide forms at the point where the dissolved oxygen and aluminum content reach point “c.”
The total aluminum content in the steel corresponds to the dissolved oxygen (O%) in the steel. The oxygen that enters the aluminum oxide inclusion remains in the molten steel and corresponds to point “b.”
The deoxidation process from the “a” to the “c” point reacts 2Al+3O=Al2O3 according to the chemical equation.
Note: Generally, the difference between full aluminum and acid soluble aluminum (% Als) is small and difficult to measure.
The relationship between the inclusion content and the total oxygen content (% Ot) in steel is established through literature. Low inclusion and total oxygen content require careful measurement, as illustrated in Fig. c.
The total oxygen analysis involves determining the density of oxide inclusions in the chemical analysis sample and counting them using SEM (Scanning Electron Microscopy).
Suitu and colleagues studied the formation of alumina inclusions in a laboratory investigation of supersaturated oxygen.
The use of Si (silicon) as a deoxidizer does not present any issues in steelmaking. However, in actual smelting operations, heterogeneous nucleation is abundant and the conditions observed in the laboratory cannot be replicated at industrial production sites.
The microstructure of aluminum oxide as an inclusion core and its growth in steel is significant to study. This is why aluminum-killed steel is crucial for large-scale industrial production.
Several authors have investigated the microstructure of aluminum oxide, its relationship with supersaturated oxygen in steel, and the impact of smelting time in detail.
Fig. 4, presented by Steinmetz and his collaborators, displays the inclusion shape corresponding to the typical deoxidizer and oxygen activity. The figure suggests that supersaturated dissolved oxygen plays a crucial role in the morphology of inclusion structure.
Recently, Tiekink and collaborators attempted to observe the functional relationship between the aluminum oxide inclusion structure, supersaturated oxygen, and aluminum composition, as shown in Fig. 5. This effort is quite complicated.
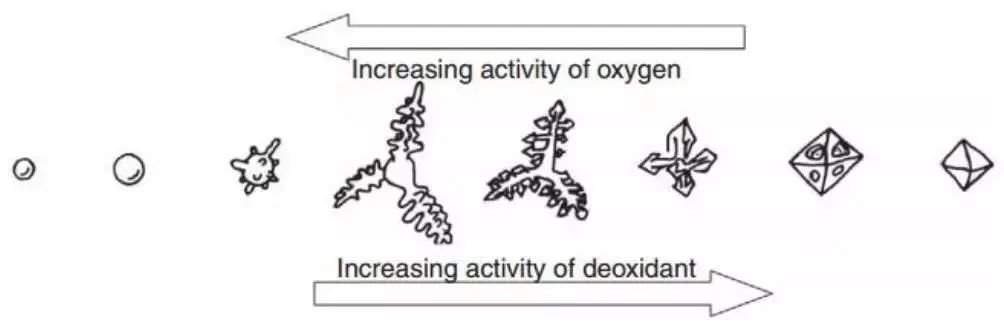
Fig. 4 Functional relationship between regional oxygen activity, aluminum activity and oxide growth
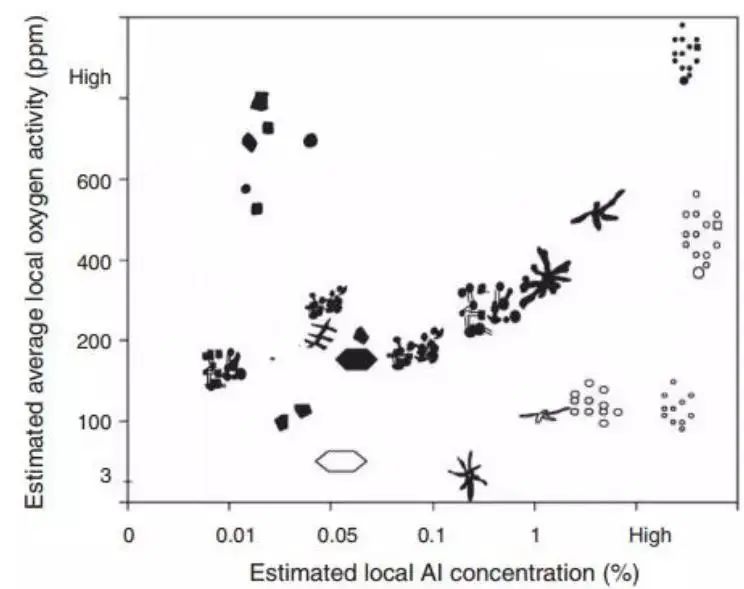
Fig. 5 Overview of morphology of alumina inclusions corresponding to different oxygen activities and Al content in steel
The structure of oxide inclusions has a major impact on the properties of the final product. It is important to note that the inclusions formed and grown in the early stages of liquid steel have distinct morphological structures, as shown in Figures 4 and 6, due to the effect of inclusions on one another (as illustrated in Fig. 7). If the refining time is prolonged, the inclusion shape will change as a result of surface energy.

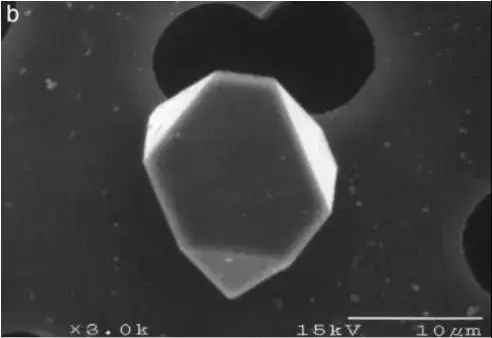
Fig. 6 Structure of some alumina inclusions.
The inclusions extracted from the casting billet matrix are dissolved. The aluminum oxide tree structure is represented by the dotted line a. A fibrous filter element is utilized to retain the inclusions during the dissolution process and acts as the backdrop for the inclusions.

Fig. 7 Alumina cluster sampled from ladle, deeply corroded with picric acid
3.1.2 Removal of inclusions
The flotation of non-metallic inclusions in a static molten steel bath can be calculated using a simple method based on the limitations of Stokes law. At normal ladle depths, the rate of floating for small particle inclusions is restricted, and it takes a considerable amount of time for them to reach the surface of the steel slag. This extended floating time is not feasible, especially for aluminum oxide inclusions. However, the impact polymerization between inclusions helps them to float, making their clustering polymerization critical.
The significance of this upward aggregation has been observed online by Emi and his colleagues. They observed the behavior of inclusions at the interface between steel and gas and found that aluminum oxide clustering occurs quickly under these conditions. On the other hand, calcium aluminate inclusions are challenging to aggregate, and complete collision only occurs in the liquid.
Wikstrom and his collaborators expanded upon the online observation of the steel slag surface and inclusions in slag and confirmed Emi’s findings at the steel slag interface. Emi and his colleagues also noted that when the phenomenon occurs at the gas-steel interface, such as on the surface of bubbles, it does not indicate directly how the liquid steel gathers into clusters, which is particularly significant for liquid inclusions. Other forces may be relevant in this case.
Regardless of whether the inclusion is solid or liquid, it plays a crucial role in clustering polymerization. For a long time, it was believed that stirring promotes the agglomeration of inclusions, but the most important factor for inclusions is to submerge them in the refining slag and refractory of the ladle wall. Lindskog and his collaborators used radioactive tracer to test and track this crucial inclusion into the refining slag and ladle wall.
Due to current limitations, BaO is the only suitable tracer that can be used to evaluate the final captured refining slag and mold flux in steel and their impact on steel cleanliness. The use of BaO tracers is highly effective in determining the effect of ladle refractory corrosion on the cleanliness of the heat number steel.
IRSID has developed the use of the element lanthanum as a tracer for oxide inclusions. La2O3 is very stable and when added to steel, alumina inclusions that already exist can be identified by lanthanum. Exogenous inclusions, which originate from mold flux, can be traced using alkaline oxides. Mold flux is typically used only in the continuous casting process and contains noticeable alkaline oxides.
Most inclusions removed during the ladle refining period are deoxidized products and go through three stages: separate inclusion production/clustering, movement towards the refining slag or refractory wall of the ladle, and absorption by the refining slag and refractory. The movement of inclusions has two crucial factors: stirring of the molten pool and being carried up by rising bubbles.
Most ladle refining results show that the size of argon stirring bubbles in the ladle is too large to effectively remove inclusions and reduce them in steel, unless a large amount of argon is used. However, Zhang and Taniguchi’s calculations indicate that argon stirring is effective when the flow velocity of molten steel is high and the bubbles are small.
The SEN submerged nozzle and ladle long nozzle of continuous casting have a noticeable effect in preventing secondary oxidation, and some advantages have also been observed in RH vacuum treatment riser steel flow. Ladle stirring promotes the inclusion to float up into the slag in clusters. Research has shown that increasing the stirring power (by using electromagnetic stirring) means increasing the kinetic energy constant for removing the inclusion (as measured by the total oxygen content in the steel).
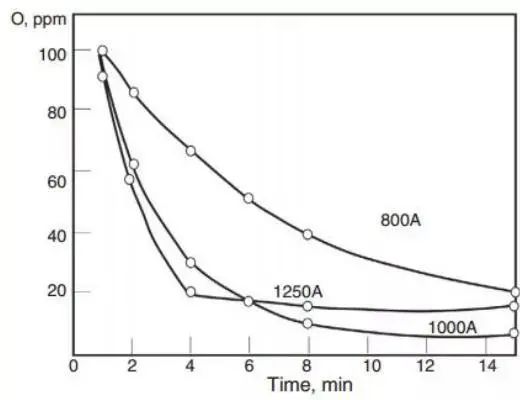
Fig. 8 Total oxygen content in ASEA-SKF ladle refining furnace is a function of stirring current and processing time.
The final oxygen content is determined by the residual aluminum content in each furnace.
Industrial observations suggest that inclusion removal will reach its maximum value at a specific mixing energy.
Suzuki and his team were the first to report this observation.
Their findings are presented as a function of the mixing energy’s specific work, highlighting the significance of mixing energy. The reduction in the effectiveness of refining to remove inclusions is likely due to the addition of refractory to the steel after corrosion, or the wrapping of steel with slag, as CaO and MgO type inclusions increase under strong stirring. These results are illustrated in Figure 9.
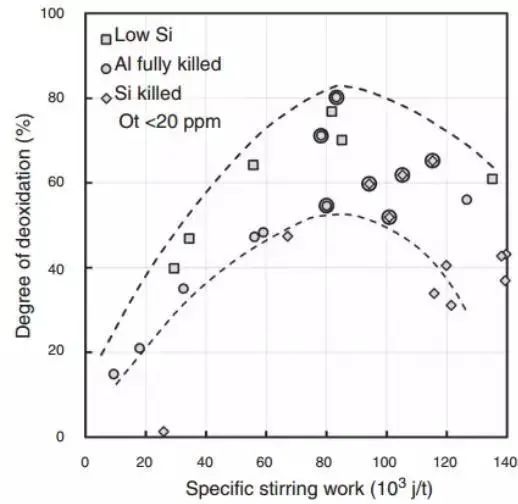
Fig. 9 The influence of mixing power on the degree of secondary oxidation. The circle point has reached below 20ppm total oxygen content
Later, Neifer and his team, along with Ek and their team, used computational fluid dynamics and physical models to investigate the removal of oxide inclusions. The relationship between the flow rate of argon in the ladle and inclusion removal was treated as a functional relationship.
The results of Neifer’s model indicated that the efficiency of removing metal inclusions was improved through the optimization of gas flow. However, they observed that increasing the gas flow rate had no effect on reducing the total oxygen content in the steel, which they attributed to secondary oxidation of the molten steel in contact with the atmosphere. These conclusions are in line with the findings of the Suzuki team.
The Ek team found that the influence of argon flow rate on inclusion removal was quite low and suggested using a lower flow rate to remove inclusions and clean the molten steel. However, the Neifer team’s industrial measurements indicated that the total oxygen content in steel decreased with an increase in gas flow. They recommended using natural convection transport in industrial experiments to achieve optimal results. However, due to the limited measurement data in industrial field ladle tests, it is challenging to draw definitive conclusions.
Recently, Zhang and Thomas collected many kinetic constants for use in the functional relationship between oxide inclusion removal and stirring power, as shown in Figure 10. They gathered measurement data and attempted to determine the optimal mixing scheme. They also performed numerical simulations to reproduce the expected behavior data of the surrounding part in Figure 10.
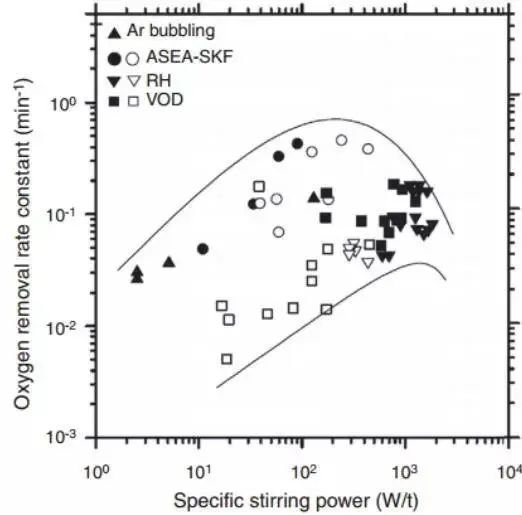
Fig. 10 Oxygen removal constant is a function of stirring power in different secondary metallurgical reaction vessels in d% Ot/dt=- kt formula
The Suzuki team highlighted that optimizing the mixing process may result in secondary oxidation. Excessive mixing may lead to slag opening on the top of the ladle, exposing the molten steel to the atmosphere, and causing slag coating at the edge of the opening.
Figure 11 illustrates the change in the chemical composition of non-metallic inclusions during the desulfurization process with strong stirring. The presence of Ca and Mg in the inclusions confirms that the slag has been emulsified.
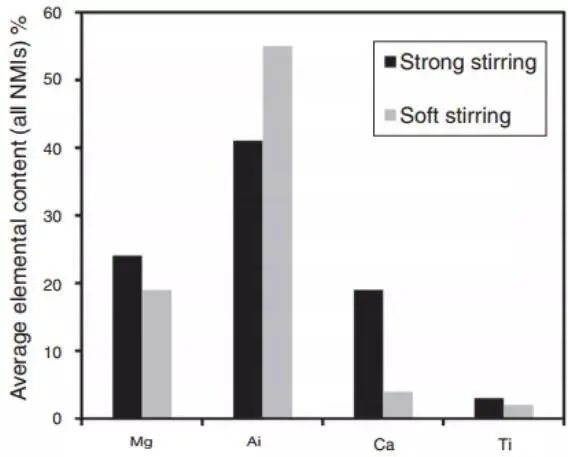
Fig. 11 The relationship between the average composition of all non-metallic inclusions and the stirring intensity was analyzed by sampling from the refining furnace, crystallizer and slab.
Kaushik’s team found that strong stirring enhances the emulsification of slag during desulfurization. The proof was in the high calcium content in inclusions. In the case of excessive argon stirring and low top slag, aluminum oxide inclusions are regenerated. Therefore, it is crucial to optimize stirring power to eliminate inclusions during the refining of clean molten steel.
The significance of secondary oxidation for the cleanliness of steel cannot be overstated. Nadif’s team reported the importance of controlling secondary oxidation. Steelmaking plants have taken various measures in recent decades to regulate secondary oxidation after refining.
The submerged nozzle SEN and the ladle nozzle are commonly used to isolate the atmosphere in continuous casting of slabs. In the production of long products, the tundish and crystallizer are protected by inert gas, which has become the norm for producing high-quality steel grades.
Special consideration should be given to the design of the inert gas valve to prevent air intrusion caused by negative pressure in the valve system. The surface tension contribution of inclusions attached to refining slag is the highest, followed by the slag’s ability to dissolve inclusions.
Regardless of the composition of the slag, most refining slag and inclusions are wet due to the surface energy difference between inclusions and molten steel, and inclusions and refining slag. This phenomenon has been discussed for many years and has been summarized by Olette. The liquid fraction in refining slag promotes the removal of non-metallic inclusions, which was known from early literature and confirmed by experiments.
However, there are still some inconsistencies in slag viscosity. Nakajima and Okamura proposed a model to explain the process of inclusions passing through the steel-slag interface. Later, many studies further discussed the subject of inclusion absorption by slag. Nakajima and Okamura suggested that under certain conditions, inclusions enter slag from steel, which may include a metal film from the interface as a channel, while in other cases, especially with solid inclusions, no such metal film exists, as shown in Fig. 12.
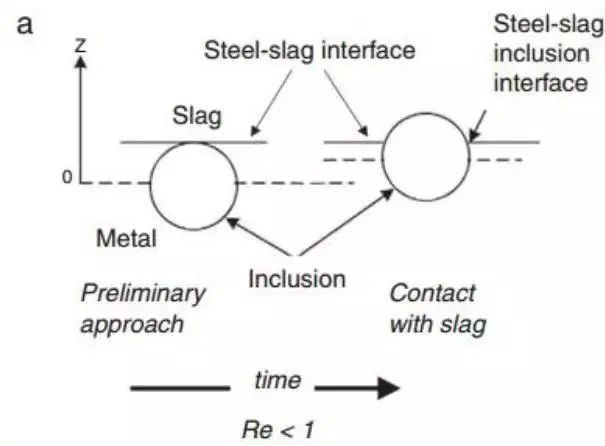
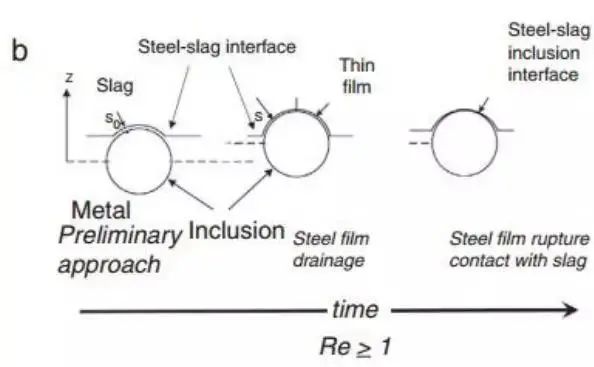
Fig. 12 shows two types of inclusions crossing the steel slag interface, introduced from Nakajima. The Reynolds number of inclusions reaching the interface determines their behavior
The conclusion of the Sridhar team is that the viscosity of slag and the associated surface energy are crucial factors in determining the passage of inclusions through the interface and reducing the likelihood of them returning to the molten steel. This is summarized by the Reynolds number when the inclusion is close to the interface.
Recently, the team observed the flow channel of this film online, which is a common occurrence. In most cases, the path of the inclusion entering the slag is extended. Once it leaves the molten steel, the liquid inclusion is immediately dissolved in the slag.
By observing online, the thermodynamics of solid inclusion dissolution can be studied experimentally. In some cases, the dissolution is controlled by transport (diffusion in the boundary layer), while in others, such as MgO inclusion, the intermediate layer formation depends on the chemical composition of the slag and may hinder dissolution at various chemical stages in refining. This was confirmed by results obtained from previous common technical methods.
Recently, the Yan team estimated the dissolution of MgO in the slag and found that all the data was controlled by the quality transmission.
The Holappa team studied the activity of the tundish covering agent, which is crucial in absorbing inclusions. They observed a complex interaction between the chemical composition, thermodynamic conditions, surface tension, and viscosity of the slag when solid non-metallic inclusions are dissolved. The team concluded that further systematic research is needed to gain a deeper understanding of this field and to develop optimization methods.
It’s good that non-metallic inclusions are adsorbed on the refractory surface of the ladle, but these inclusions may also become the source of inclusions in the next furnace, depending on the composition of the ladle slag.
If inclusions are adsorbed in the molten steel pipeline channel, it can cause significant problems, such as long treatment time and high cost due to nozzle blockage in the continuous casting process. This nozzle clogging phenomenon is well described in the references.
It’s noted that the flocculent flow at the nozzle is caused by the adhesion and accumulation of aluminum oxide inclusions and FeO, which may be formed in the secondary oxidation. This phenomenon is clearly described in the references.
There is a vast body of literature on the absorption of primary inclusions by mold powder during both continuous casting and ingot casting. The consensus among these sources is that this phenomenon is indeed possible.
The mold flux used in continuous casting and mold casting (which is similar to the tundish covering agent) must have multiple functions and possess fluidity. However, it is subject to various constraints within the mold, such as avoiding the inclusion of mold flux into the surface of the primary green shell. This, to some extent, restricts the movement of inclusions and keeps them within the mold flux. At the same time, it optimizes the flow of molten steel in the tundish and mold, allowing for the molten steel to reach the slag interface with inclusions. However, this creates conflicting objectives with other relevant metallurgical goals.
The best way to remove inclusions caused by convection is through ladle refining, and it is also crucial to prevent secondary oxidation from creating new inclusions, which is a crucial aspect of producing clean steel.
Another issue is the movement of primary inclusions in the continuous casting slab. It has been widely acknowledged that inclusions are asymmetrically distributed in the cross section due to the arc continuous casting process. This asymmetry is often related to the clogging of floc flow at the nozzle.
Sichen recently demonstrated the impact of the secondary refining model, particularly the refining furnace process. The model seeks to explain the interface reaction of steel slag, the opening of the transition stirring slag layer, the generation, nucleation, growth, separation, and floatation removal of inclusions, utilizing most of the available technologies.
However, Sichen pointed out that the main variables in the ladle refining process, such as mass transfer efficiency, inclusion floating removal rate, the opening of the over-stirring slag layer, and argon flow rate, are difficult to simulate due to uncertainties in industrial production, such as the ladle vent plug and gas pipeline leakage.
It is challenging to control and detect argon flow velocity in industrial ladle refining. Camera and image analyzer technology can be used to monitor the opening of the ladle slag layer, while vibration measurement can be used to control argon flow. These technologies have already been adopted by some steel plants.
During solidification, the driving force of secondary inclusion precipitation increases the segregation of solute elements, and the solubility of oxides and sulfides in steel decreases as the temperature drops.
The phenomenon of inclusion precipitation due to changes in steel solubility has been a topic of discussion for some time.
Since the 1960s, the terms “primary” and “secondary inclusions” have been established, and the relationship between segregation and inclusion precipitation has been defined.
At that time, the first model explaining this process was introduced.
Turkdogan and Flemings made a significant contribution to our understanding of the overall impact of solubility changes with decreasing temperature on secondary inclusion segregation.
During the 1980s and 1990s, Nippon Steel and IRSID developed advanced models, which were later applied to the precipitation of nitride in HSLA microalloyed steel during solidification.
These models paved the way for the study of inclusion engineering.
Today, we know that by combining a thermodynamic database and a kinetic database, we can simulate solidification and calculate inclusion formation.
These calculations begin with the desired chemical composition of steel, predict the precipitation of inclusions, and guide the design of refining slag composition during ladle refining to produce clean steel.
The interaction between liquid steel, dendrites, and inclusions formed at the front of solidification is an important area of study.
In-line observations indicate that solidification conditions play a crucial role in the formation of inclusions that are pushed into the liquid phase at the interface and engulfed by inclusions.
In theory, these results can be calculated and adjusted to take into account the effects of surface tension and density.
The focus of theoretical research is mainly on the composition of metal matrices, and the findings regarding non-metallic inclusions in steel are also more in line with actual conditions.
The findings indicate that the critical growth velocity (V) can be represented as V = k/R, where R is the radius of engulfment and repulsion of the inclusion interface, and k depends on the type of inclusion.
The structure of secondary inclusions is significantly impacted by the reactions that occur during precipitation, with the precipitation of carbides being one of the best examples.
Since Sims first observed the impact of re-oxidation on sulfide structure in 1930, he later proposed three distinct types of sulfides, which have been thoroughly described by various authors.
Recently, the Ishida team has highlighted that, besides the type of reaction accompanying sulfide formation, the surface tension also plays a crucial role in shaping sulfide structure.
The Gaye team has presented the most comprehensive and insightful explanation of the thermodynamic application of inclusion engineering in steel.
Figure 13 provides a concise illustration in two adiabatic ternary phase diagrams.
Once the necessary inclusions have been identified, the chemical composition of the steel that will produce these inclusions can be determined.
The composition of the refining slag used for refining can then be calculated based on the chemical composition of the steel through a steel slag balance.
The saying, “Steel can only be made after smelting slag” is well-founded and has been successfully applied in the production of various types of steel.
In steel production, it is important to avoid hard phase aluminum oxide composite inclusions (such as spinel).
For example, in the production of bearing steel, the inclusion acts as a nucleation core during the phase transformation that occurs during cooling.
Calcium treatment transforms inclusions into liquid inclusions and, along with calcium, modifies sulfides to avoid nozzle clogging.
While the process of inclusion modification may seem straightforward, it is also a topic of discussion in this chapter.
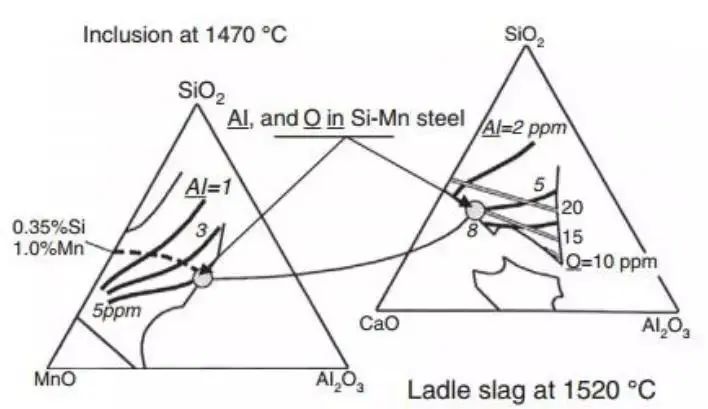
Fig. 13 shows the transformation process of inclusions. From the thermodynamic point of view, low melting point Al2O3 inclusions are expected to be obtained in Si Mn killed steel.
The expected inclusions are shown in the simplified phase diagram of MnO-SiO2-Al2O3 ternary system.
The diagram on the left displays the system at the 1470 ℃ isotherm. The region is indicated as 100% liquid phase, confined within the thin line, as depicted in the figure.
The solid and thick isopleth at the specified temperature represents the aluminum content in molten steel with liquid inclusions in equilibrium. The dotted line, on the other hand, indicates the chemical composition of the inclusions in 0.35% Si, 1% Mn steel at a particular temperature, varying with the aluminum content of the steel grade.
As per the diagram, if one wants to have liquid inclusions, the aluminum content of the steel should not exceed the gray circle (8ppm).
The figure on the right shows the simplified ladle refining slag of CaO-SiO2-Al2O3 system.
The 1520 ℃ isotherm represents the conditions in the ladle refining furnace and shows that the 100% liquid inclusion region is confined to the fine solid line.
At the chosen temperature, the coarse solid line represents the aluminum content of the steel in equilibrium state within the slag system. The grey line displays the corresponding oxygen content in the steel under examination.
If liquid inclusions are desired (on the left side of the diagram), the refining slag composition must be chosen as indicated by the diagram to ensure that the aluminum content in the steel is less than 8ppm.
The tire radial steel wire is made of high-carbon steel that is deoxidized using silicon-manganese.
Similar methods are also used in the manufacture of many automotive spring steels.
Brittle non-metallic inclusions, typically aluminum oxide inclusions or inclusions with high aluminum oxide content, have a significant impact on both the drawing performance of the steel wire and the quality of the spring steel.
To prevent the formation of aluminum oxide inclusions or inclusions rich in aluminum oxide, the composition of the steel must be adjusted. This involves strict control of the aluminum oxide content in the slag, monitoring of raw and auxiliary materials to prevent aluminum from entering the steel, and the use of a low-alkalinity binary slag system.
This solution was initially contradictory to the prevalent refining operations of the time.
There are numerous excellent examples and articles that address the inclusion treatment control thermodynamics of tire radial and spring steel.
The impact of a single type of inclusion on the fatigue life of bearing steel remains a topic of debate. However, it is widely accepted that the size and amount of inclusions in steel significantly affect the fatigue life of bearing steel.
It is well known that calcium aluminate and spinel inclusions negatively impact the performance of bearing steel.
As a result, some believe that the production of bearing steel should aim to achieve a very low total oxygen content and very low sulfur and aluminum content, to keep the non-metallic inclusions at a minimum.
Additionally, the introduction of magnesium from slag can lead to the formation of spinel inclusions, which should be avoided.
To produce high-quality bearing steel, various steel plants adopt different process methods based on their specific conditions.
However, controlling the chemical composition of refining slag is always a critical factor in controlling non-metallic inclusions in bearing steel.
Figure 14 demonstrates the influence of Al, O, and Ag contents in 100Cr6 (AISI52100) bearing steel on the composition of slag. The comparison of calculated and measured aluminum and oxygen contents in steel is also shown.
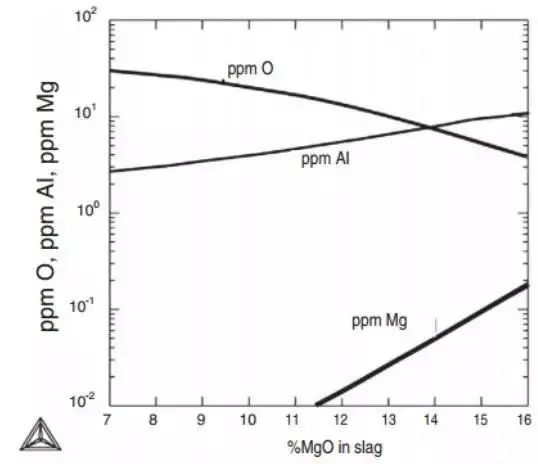
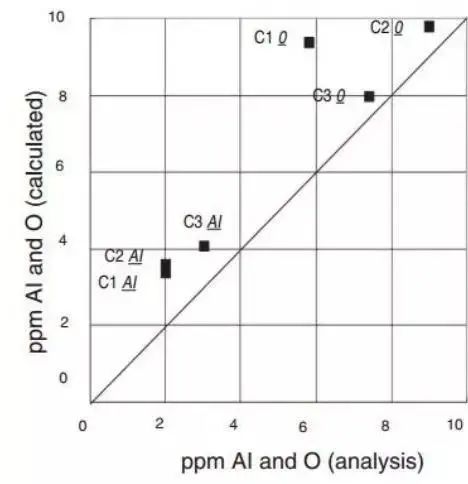
Fig. 14
a. Under the equilibrium state of bearing steel, the refining slag% Al2O3=5%,% CaO=48% remain unchanged, and the influence of MgO on Al, O and Mg is calculated using Thermo calc and SLAG2 databases at 1540 ℃.
b. Compare the calculated value and measured value of bearing steel after finishing the refining of furnace 3, and Thermo calc® and SLAG2 database is used for calculation.
Calcium treatment is used to eliminate sulfide inclusions and regulate the anisotropy of hot-rolled materials or forgings. It also helps to improve the workability of inclusions.
The practice of using calcium treatment to transform aluminum oxide inclusions into liquid composite inclusions to prevent clogging of nozzle flocs has gained widespread use in recent decades, despite being a controversial method.
The process of calcium treatment is complex, requiring consideration of factors such as calcium solubility, yield, and high vapor pressure caused by oxidation during calcium addition. These factors have been thoroughly researched.
Studies have also been conducted on the mechanism of inclusion denaturation and the ideal amount of calcium required to achieve the desired outcome.
The formation of inclusions is a complex process. The outer layer, often composed of oxides, is covered with a sulfur-rich compound coating. This phenomenon and the distribution of individual elements are illustrated in Figure 15.
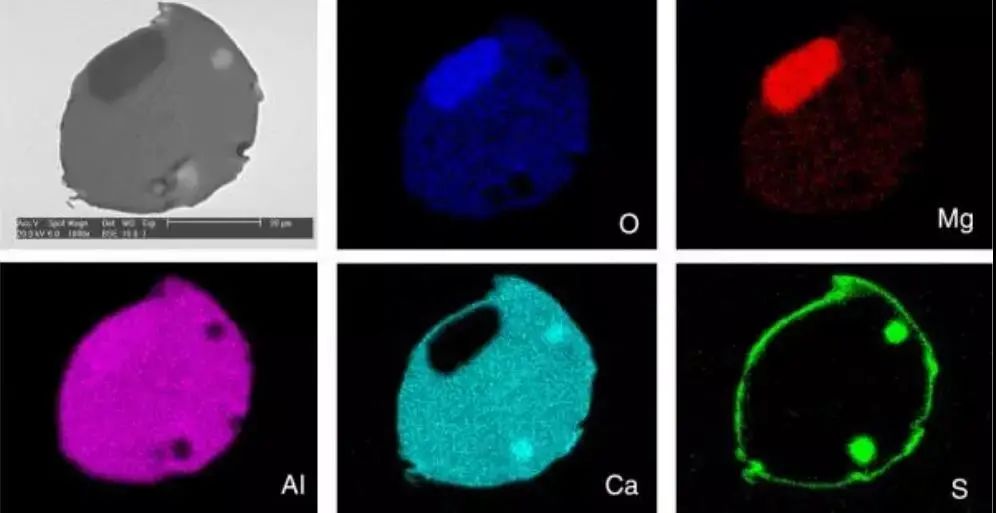
Fig. 15
Calcium treatment is used to improve the castability of steel. It dissolves the large particle inclusions composed of calcium aluminate, sulfide, and AgO found in the slab during continuous casting.
Magnesium is also reduced from the slag into the steel during the treatment process.
As a result of the treatment, a significant portion of the inclusions become a liquid phase and will not clog the nozzle during casting.
However, if the temperature of the molten steel is too low, casting will become challenging.
The reaction of non-metallic inclusions formed during solidification is a complex process, as illustrated in Figure 16.

Fig. 16
The slab sample contains broken large calcium aluminate inclusions with complex phases, and the inclusion shell displays a dendritic solidification structure.
The amount of calcium needed to modify calcium oxide inclusions is dependent on the total oxygen content in the steel.
Unfortunately, there is no current method to accurately determine the total oxygen content in the steel in real-time, making it difficult to determine the appropriate amount of calcium to add.
This presents a significant challenge for industrial production.
One solution is to use thermodynamics to understand the clogging of floc flow in the nozzle and establish the castable window for continuous casting.
The level of dissolved oxygen can be measured, and this data can also be used to monitor the efficiency of the calcium treatment, as demonstrated in Figure 17.
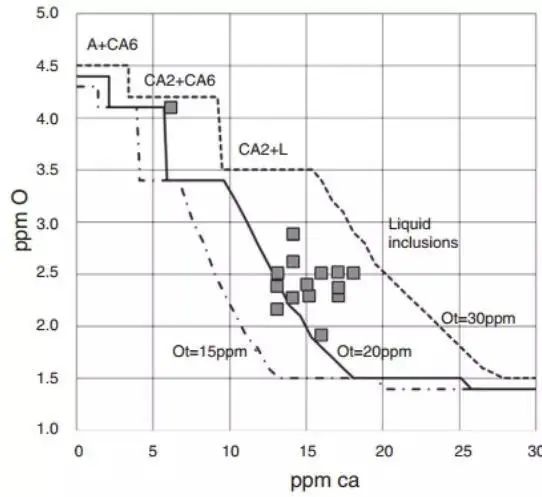
Fig. 17
The figure above displays the correlation between the calcium content and the calculated dissolved oxygen in steel with a composition of 0.025% Al, 0.01% S, and varying total oxygen contents of 20, 25, and 30 ppm from left to right, at a temperature of 1540 ℃.
The presence of non-metallic phases is indicated within each composition range.
The calculations were performed using the Thermo-Calc® and SLAG3 databases.
Each point represents the experimental measurement of the dissolved oxygen content in steel, which was obtained without any blockage in the nozzle.
The final casting process is encountering a challenge that requires controlling the microstructure and cannot solely rely on hot rolling.
Research has shown that the presence of ferrite in the weld metal positively impacts the formation of non-metallic inclusions.
The theory of inclusion nucleation suggests that inclusion formation depletes Mn in the surrounding matrix, which appears to be effective.
Non-metallic oxide inclusions serve as the nucleation cores for MnS inclusions and have produced favorable outcomes in applications.
Additionally, secondary oxidation of titanium in silicon manganese steel transforms inclusions into titanium oxide, while oxide and nitride have high nucleation efficiency in ferrite, as confirmed.
Koseki, Inoue, Suito, and Park have proven that titanium nitride can effectively act as a nucleating agent, promoting the appearance of large equiaxed grains in continuous casting stainless steel and welding processes.
Park and Kang have recently made advancements in this field.
Thermodynamic calculations and model simulations show that alloy design and process design in oxide metallurgy can be highly beneficial.
In recent decades, the iron and steel industry has encountered a challenge in accurately classifying and quantifying non-metallic inclusions through traditional comparison charts and images. To improve the quantitative analysis of inclusions, including information on size, volume fraction, and composition, new methods have emerged.
In many cases, multiple methods must be employed simultaneously to gain a comprehensive understanding of the nature and process of non-metallic inclusions. Research has shown that certain characteristics depend on the distribution of inclusions, while others depend on other factors. For instance, the fatigue performance of steel is influenced by the size of the largest inclusion.
The cleanliness of steel products varies widely, except for low-end products. The total oxygen content of low carbon aluminum killed steel (LCAK) is approximately 40 ppm, while typical bearing steel has a total oxygen content of around 5 ppm. The volume fraction of oxide inclusions is significantly different, but the presence of sulfide inclusions is not mentioned.
Extreme value statistics and its application play a critical role in fatigue analysis. These methods are not widely covered in general literature, but are included in this literature review with references provided for further reading. The grade method of inclusion evaluation using extreme value statistics, proposed by Murakami in the program, has been widely used in the field of fatigue and has produced excellent results.
It is important to note that this method does not take into account the maximum inclusion size in fatigue analysis. In fact, the volume fraction of inclusions caused by large particle inclusions may increase. This aspect of the method may not align with the expectations of steelmakers, as it does not consider the inclusion of the largest particle.
The well-established saying “Making good slag leads to making good steel” has been deeply ingrained in the steelmaking industry.
Over the past few decades, the recognition of the impact of non-metallic inclusions on steel properties has moved the steelmaking process from merely preventing inclusion contamination to optimizing the composition, quantity, and distribution of inclusions in steel.
This transformation impacts every aspect of the steelmaking process, from raw material selection (such as avoiding aluminum contamination), to slag composition design, secondary refining conditions optimization (such as refining process time and hydrodynamic conditions), and careful tundish and mold operation control.
It has become standard practice in the production of various steel grades to carefully control secondary oxidation in all processes.
Thermodynamics plays a critical role in researching the influence of inclusions on steel. Understanding thermodynamics, the chemical composition of steel and refining slag, and the interactions between steelmaking process conditions are now widely studied.
There has also been a significant improvement in modeling tools, allowing for a more science-based approach to controlling inclusions in steel.
These technologies have been widely adopted and continue to evolve in the field of non-metallic inclusion modification. However, there is still a need to continually improve refining slag and fully understand the role of non-metallic inclusions in steel.
Inclusion modification and oxide metallurgy engineering are now widely used in steel plants, resulting in steel that is at least an order of magnitude cleaner than it was several decades ago. This has also presented new challenges for the qualitative and quantitative analysis of non-metallic inclusions.
Quantitative analysis of all inclusions and their impact on steel properties and behavior is now a basic requirement, and there is ample room for discussion and future research.
Despite the advancements and insights summarized in this review, the ongoing challenge in the coming decades will be to continuously improve various technologies and improve the quality of steel.

As the founder of MachineMFG, I have dedicated over a decade of my career to the metalworking industry. My extensive experience has allowed me to become an expert in the fields of sheet metal fabrication, machining, mechanical engineering, and machine tools for metals. I am constantly thinking, reading, and writing about these subjects, constantly striving to stay at the forefront of my field. Let my knowledge and expertise be an asset to your business.