
Have you ever wondered why some metals weld seamlessly while others crack and break? This article explores the fascinating world of weldability, focusing on carbon steel and its various forms. Learn how carbon content, impurities, and welding methods impact the strength and durability of welded joints. Get ready to uncover the secrets of successful welding!

Weldability refers to the ability of a material to be welded into components that meet the specified design requirements under defined construction conditions and to satisfy the predetermined service requirements.
Weldability is influenced by four factors: material, welding method, component type, and usage requirements.
Iron-Carbon alloy is a binary alloy composed of iron and carbon. It is the most widely used type of iron-based material. Carbon steel and cast iron are materials of the iron-carbon alloy. Alloys with less than 0.0218% carbon are known as industrial pure iron. Iron-carbon alloys with less than 2.11% carbon are referred to as steel.
Alloys with more than 2.11% carbon are known as cast iron. Besides carbon, carbon steel and cast iron contain impurities like silicon, manganese, sulfur, phosphorus, nitrogen, hydrogen, and oxygen; these impurities can affect the properties of the steel.

1. Classification of Carbon Steel
(1) Based on Carbon Content
(2) Based on Steel Quality
(3) Based on Steel Usage
(4) Based on Steel Deoxidation Levels:
Designation Method
Q235-AF
Q235-AF signifies a carbon structural steel with a yield point of ≥235MPa, belonging to grade A boiling steel.
Quality grades are as follows:
The properties of carbon steel are primarily determined by its carbon content. The correlation between carbon content, weldability, and the structure and performance of carbon steel can be seen in Figure 2-1 and Table 2-1.
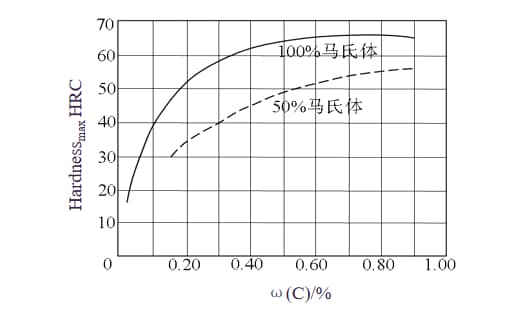
Table 2-1: Weldability of Various Types of Carbon Steel
| Name | Carbon Content | Typical Hardness | Typical Uses | Weldability |
| Low Carbon Steel | ≤0.15% | HRB | Special plates and profiled thin sheets, strips, and welding wires | Excellent |
| 0.15%~0.25% | 30-35HRB | Structural profiles, plates, and bars | Good | |
| Medium Carbon Steel | 0.25%~0.60% | 42-46HRC | Mechanical parts and tools | Average (typically requires preheating, low-hydrogen welding method recommended) |
| High Carbon Steel | >0.60%-1.00 | 55HRC | Springs, molds | Poor (requires low-hydrogen welding method, preheating, and post-heating) |
While the strength of carbon steel is relatively low, it is easy to smelt, has excellent processability, and is inexpensive. It boasts superior forgeability, weldability, and cutting performance. Carbon steel is usually supplied to the market in various shapes and sizes, such as round steel, square steel, I-beam steel, and rebar.
Low carbon steel, with its carbon content as low as 0.25% or less, and minimal M and Si contents, has a low hardening tendency, making it the most weldable type of steel. Besides C, M, and Si, impurities like S, P, O, N in carbon steel can impact its mechanical properties, cold cracking of weld joints, hot cracking, and sensitivity to age brittleness. The mechanical properties of common low carbon steel are as shown in Table 2-2.
Table 2-2 Mechanical Properties of Low Carbon Steel
| Grade | Level | Tensile Test (Not Less Than) | Impact Test | Cold Bending Test 180°, B=2a | ||||
| Yield Point /MPa | Tensile Strength /MPa | Elongation Rate | Temperature /℃ | Charpy V-Notch Impact Absorption Energy (Longitudinal) /J≥ | ||||
| Bending Core Diameter d | ||||||||
| Longitudinal Specimen | Transverse Specimen | |||||||
| Q195 | – | 195 | 315-430 | 33 | – | – | 0 | 0.5a |
| Q215 | A | 215 | 335-410 | 31 | – | – | 0.5a | a |
| B | 20 | 27 | ||||||
| Q235 | A | 235 | 370-500 | 26 | – | – | a | 1.5a |
| B | 20 | 27 | ||||||
| – | 0 | |||||||
| D | -20 | |||||||
| Q275 | A | 275 | 410-540 | 22 | – | – | 1.5a | 2a |
| B | 20 | 27 | ||||||
| C | 0 | |||||||
| D | -20 | |||||||
| Q245R | – | 245 | 400-520 | 25 | 0 | 31 | 1.5a | |
| (20g,20R) | ||||||||
Applications:
Generally, no heat treatment is conducted after forming. Most of them are used directly in a hot-rolled state.
Carbon steel and low-alloy steel are categorized based on their chemical compositions into three groups: carbon steel, low-alloy steel, and alloy steel, with carbon steel (commonly referred to as carbon steel) being the most widely used.
Apart from carbon, which is the primary alloying element in carbon steel, there are also silicon (Si<0.5%), manganese (Mn<0.8%), and inevitable impurities such as sulfur and phosphorus.
Low-alloy steel is derived from carbon steel, and one or more alloying elements are intentionally added to obtain certain properties. For example, 16Mn steel.
Classification and Usage of Alloy Steel:
Total alloying element content:
1. Weldability Analysis of Low Carbon Steel
The weldability of carbon steel deteriorates with an increase in carbon content. When selecting welding materials, not only should they match the parent material in composition and properties, but harmful elements such as sulfur and phosphorus should be avoided from being introduced into the weld metal.
When welding carbon steel with a carbon content higher than 0.25%, the source of hydrogen should be minimized.
The mechanical reasons for cracks when welding carbon steel are structural constraint stress and uneven thermal stress. Different technological measures should be taken depending on the carbon content.
For low carbon steel, special attention should be paid to prevent cracks caused by structural constraint stress and uneven thermal stress. In addition to preventing cracks caused by these stresses, high carbon steel should especially avoid cracks caused by hardening.
The weldability of carbon steel primarily depends on its susceptibility to cold cracking, hot cracking, and the toughness of the joint. The carbon content of the steel and the deposited metal has the most significant impact on the cold cracking of carbon steel.
Carbon Equivalent: CE=C+Mn/6+Si/24
For carbon steel, the silicon content is relatively low, not exceeding 0.5%. Sometimes, its impact can be overlooked. As the Carbon Equivalent (CE) value increases, the propensity for cold cracking increases, and weldability deteriorates. Typically, when the CE value exceeds 0.40%, the sensitivity to cold cracking increases.
The tendency for hardening of the weld and heat-affected zones, and their susceptibility to cold cracking, are not only related to composition, but the structure’s impact on performance is even more significant. Given a certain composition, the structure depends on the cooling rate, which can be determined through its SHCCT (Simulated Heat Affected Zone Continuous Cooling Transformation) structure. Figure 2-2 presents the SHCCT diagram of Q235 (A3) steel.
Controlling the cooling rate of the welding area is a crucial method for altering the structure type and hardness of the welding zone, thereby reducing the occurrence of cold cracking.
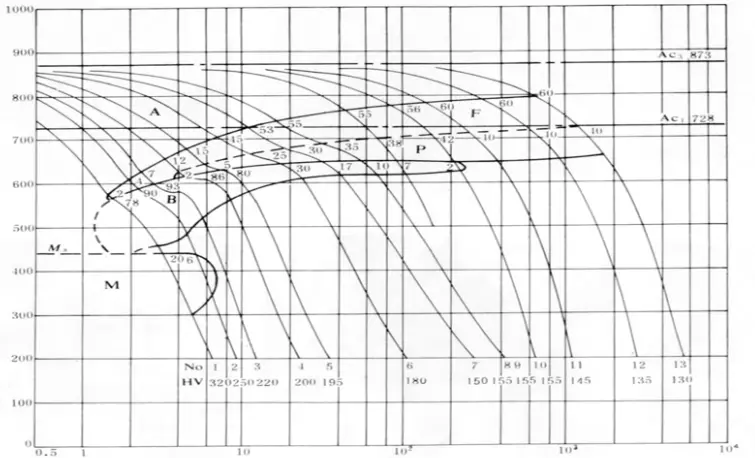
As the thickness of the weldment increases, or when the environment temperature is lower during welding, or the energy of the welding wire is smaller, the heat dissipation accelerates, and the cooling speed of the welded joint increases, which in turn increases the tendency of cold cracking.
T-joints and lap joints have a larger heat dissipation area compared to butt joints, thus accelerating the cooling rate in the welding zone and making it prone to the formation of hardened structures.
Preheating, raising the temperature between welding tracks or layers, or post-heating measures can also reduce the cooling speed during welding.
The hardening of carbon steel is primarily due to the formation of martensitic structures. Martensite is a supersaturated solution of carbon in α-Fε, and its hardness is related both to the carbon content in the steel and the quantity of martensite formed. The amount of martensite is influenced by the cooling rate; a very rapid cooling speed can produce 100% martensite, thereby achieving the highest hardness.
Hydrogen and Degree of Restraint
The hydrogen in the welding area primarily originates from the welding materials and moisture in the welding zone. The dissolved hydrogen in the weld can be reduced by using low-hydrogen welding materials, increasing the drying temperature of the welding materials, reducing the moisture content in the shielding gas, or lowering the humidity in the welding area.
An increase in the thickness of the steel plate or the rigidity of the structure will enhance the degree of restraint, thereby increasing the sensitivity to hydrogen-induced cracking.
The three major inducements of cold crack sensitivity are the hardened structure, hydrogen, and restraint stress. When the composition of the steel is fixed, the higher the proportion of the hardened structure, the lower the critical hydrogen content required to cause cold cracking, and the lower the necessary restraint stress, thus increasing the tendency for cold cracking.
When the structure and hydrogen content are fixed, the greater the degree of restraint, the higher the sensitivity to cold cracking. Therefore, in the tendency of carbon steel to cold crack, the three factors of hardened structure, hydrogen, and restraint stress mutually promote each other and are interdependent.
Hot Cracking
Hot cracking susceptibility is closely related to impurities such as sulfur (S) and phosphorus (P) in the steel. During the welding of carbon steel with high S and P content, the low melting point S and P compounds gathered on the grain boundaries in the heat-affected zone, causing liquidation cracks near the fusion line of the heat-affected zone.
For thicker steel plates, sulfides distributed along different segregation zones can lead to laminar tearing cracks in T-joints and others. When the dilution rate of the base material is high, more S and P enter the weld seam, which can easily cause hot cracking in the weld seam.
To avoid this, the joint design or process operation should prevent the weld seam from having a narrow and deep shape. Low carbon steel arc weld seams usually have a higher resistance to hot cracking.
Laminar Tearing
Boiling steel has a higher oxygen content and a noticeable segregation band in the center of the plate thickness, which can lead to cracking and porosity during welding. There is a certain tendency for laminar tearing in thick plate welding, and it also exhibits a high degree of aging sensitivity. The brittle transition temperature of the welded joint is also on the higher side.
Therefore, boiling steel is typically not used in manufacturing structures that are subject to dynamic loads or operate at low temperatures.
Changes in the Performance of the Heat-Affected Zone During Welding
The main change is in the joint’s ductility, which depends on the steel’s composition, the heat treatment state of the base material before welding, and the welding heat process. Carbon steel is mainly delivered in a hot-rolled state, but for some high-quality carbon structural steels and carbon structural steels for special purposes, the delivery state can also be controlled rolling, normalizing, normalizing + tempering, or quenching + tempering.
During the cooling process of the steel, a large deformation is applied at a lower temperature before the austenite decomposes, which increases the ferrite phase nucleation rate. The resulting grains are significantly refined, thereby drastically improving their strength and ductility.
Certain welding methods have dispersed heat sources or excessively high linear energy, such as gas welding and electroslag welding. These methods cause the grains in the coarse grain zone of the weld heat-affected area to become even larger, thereby reducing the impact toughness of the joint. Consequently, post-weld heat treatment is often required for significant structures.
In conclusion, low carbon steel has a low carbon content and a very low alloy element content. Therefore, using conventional welding methods, there will be no hardened structures or cold cracks in the joints. As long as the welding materials are selected correctly, satisfactory welding joints can be achieved.
2. Welding Methods
There are no specific requirements for choosing a welding method for low carbon steel. The selection can be made based on variables such as material thickness, product structure, performance requirements, and production conditions.
Shielded metal arc welding, CO2 gas shielded welding, and submerged arc welding are common welding methods.
3. Weldability Analysis of Medium Carbon Steel
Cold Cracking
Medium carbon steel has a higher carbon equivalent than low carbon steel. When the carbon content exceeds 0.25%, the hardenability of the steel increases. During welding, if the cooling speed is fast (as shown in the CCT diagram of 45 steel in Figure 2-3, the cooling speed exceeds the critical value point c), a martensitic structure will form in the heat affected zone.
The martensitic structure of medium carbon steel is more brittle, and under the action of welding stress, it is prone to cold cracking and brittle fracture. The heat affected zone of medium carbon steel is more likely to form a hardened martensitic structure after welding. This structure is more sensitive to hydrogen, and the critical stress required for cold cracking is lower.
Therefore, it is advisable to use low-hydrogen electrodes and appropriately increase the preheating temperature to reduce residual stress.
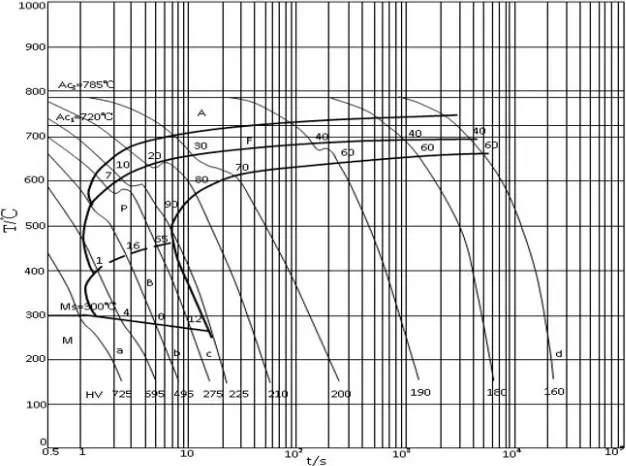
Hot Cracks
When the mass fraction of carbon in steel exceeds 0.25%, the segregation of carbon itself and its promotion of other elements like sulfur (S) and phosphorus (P) becomes significantly prominent. This is especially the case when the S and P content in the parent material is controlled near the upper limit of the qualified value required by the standard. Low-melting-point sulfides can lead to an increased tendency for hot cracking, particularly at the arc pit.
Therefore, in welding carbon steel, strict control over the mass fraction of sulfur is a must.
Pores
Due to the higher carbon content compared to low carbon steel, the amount of carbon entering the weld pool increases. This could potentially lead to the formation of CO pores during welding metallurgical reactions, thereby augmenting the sensitivity to pore formation in the weld seam.
When welding boiling steel, it is crucial to ensure that the chosen welding material contains adequate deoxidizers to prevent the formation of pores in the weld seam.
Changes in the Performance of the Heat-Affected Zone in Welding
The heat-affected zone in welding undergoes changes due to the emergence of hardened structures, resulting in increased strength, brittleness, and hardening, as well as heightened susceptibility to cold cracking. The carbon from the melted parent metal enters the weld pool, leading to an increase in the carbon content of the weld metal. The performance varies between weld paths due to differences in dilution rates.
As the carbon content in medium carbon steel increases, its weldability deteriorates. The main issues encountered during welding are hot cracking, cold cracking, porosity, and brittle fracture, and sometimes there is a decrease in strength in the heat-affected zone. The more impurities in the steel and the greater the structural rigidity, the more severe these problems become.
When welding repair work is performed on medium carbon steel castings, precautions should be taken to prevent cold cracking during welding or cracking due to excessive residual stress in the repaired area.
4. Welding Methods
Typically, stick electrode welding or CO2 gas shielded welding methods are employed. When adding wear or corrosion-resistant surfaces to medium carbon steel, or repairing larger worn surfaces on medium carbon steel, submerged arc welding can also be utilized.
5. Weldability Analysis of High Carbon Steel
Poor Weldability
High carbon steel, which includes structural carbon steel, carbon steel castings, and carbon tool steel, contains more than 0.6% carbon. The weldability of these materials is quite poor, and welding can result in hard, brittle high-carbon martensite. They have a high tendency for hardening and cracking. Given their poor weldability and high hardness, these types of steel are typically used in components or parts requiring high hardness and wear resistance, rather than in the creation of welded structures.
Welding methods: Shielded metal arc welding and gas welding are commonly used for repair welding.
Stainless steel refers to a type of steel that does not easily rust in the atmosphere; it is a steel that is more resistant to corrosion under specific conditions of acid, alkaline, and salt. Due to its excellent corrosion resistance, formability, and toughness over a wide temperature range, stainless steel is widely used in petrochemicals, nuclear energy, light industry, textiles, food, and household appliances.
1. Classification of Stainless Steel
(1) Austenitic Stainless Steel
Austenitic stainless steel is distinguished by its non-magnetic properties, good low-temperature performance, formability, and weldability.
Ferritic stainless steel is characterized by strong magnetism, easy formability, rust resistance, and pitting resistance.
(3) Martensitic Stainless Steel
Martensitic stainless steel is known for its high strength and hardness, although its corrosion resistance is slightly inferior to that of austenitic and ferritic stainless steel.
(4) Duplex Stainless Steel
Duplex stainless steel exhibits high yield strength, resistance to pitting and stress corrosion, and is easy to form and weld.
(5) Precipitation Hardening Stainless Steel
Precipitation hardening stainless steel has a chromium content of around 17%, and coupled with elements like nickel and molybdenum, it not only possesses sufficient stainless properties but also exhibits corrosion resistance comparable to austenitic stainless steel.
2. Role of Alloy Elements
Iron: It is the basic metallic element in stainless steel.
Chromium: It is the primary ferrite-forming element. When combined with oxygen, chromium forms a corrosion-resistant Cr2O3 passive film, making it the essential element for maintaining the corrosion resistance of stainless steel.
Carbon: It is a strong austenite-forming element that can notably enhance the strength of steel. However, carbon can also adversely affect the corrosion resistance.
Nickel: It is the main austenite-forming element. Nickel can slow down the corrosion of steel and the enlargement of grains during heating.
Molybdenum: It is an element that forms carbides. The carbides it forms are extremely stable, preventing grain growth during austenite heating and reducing the steel’s overheat sensitivity.
Niobium, Titanium: These are strong carbide-forming elements that enhance the steel’s resistance to intergranular corrosion.
Nitrogen: It is a strong austenite-forming element that significantly boosts steel’s strength.
Phosphorus, Sulfur: These harmful elements in stainless steel adversely affect its corrosion resistance and stamping properties.
3. General Physical Properties of Stainless Steel
(1) Heat Conduction: The heat transfer rate of stainless steel is relatively slow.
(2) Thermal Expansion: Compared to carbon steel, the linear expansion coefficient of 304-grade steel is larger.
(3) Electrical Resistance: Generally, the electrical resistance of alloys is higher than that of pure metals, and the same applies to stainless steel.
(4) Magnetic Properties of Stainless Steel
Table 3: Magnetic Properties of Various Materials
| Materials | Magnetic Properties | Magnetic Permeability :μ(H=50e) |
| SUS430 | Strong Magnetism | – |
| Iron | Strong Magnetism | – |
| Ni | Strong Magnetism | – |
| SUS304 | Non-Magnetic (exhibits magnetism during cold work) | 1.5(65% Processing) |
| SUS301 | Non-Magnetic (exhibits magnetism during cold work) | 14.8(55% Processing) |
| SUS305 | Non-Magnetic | – |
1. Weldability of Stainless Steel
The electrical resistance of stainless steel is significantly higher than that of low-carbon steel. During welding, both the welding rod and the parent material in the welding area are prone to heating and melting. This can cause the surrounding base material to overheat, resulting in uneven deformation in the welding area and coarse grains.
Stainless steel has a high linear expansion coefficient and a low thermal conductivity coefficient, making it difficult for heat to dissipate. During welding, the depth of penetration is high, and the heating from welding causes the structure to expand. During cooling, significant shrinkage deformation and tensile stress occur, which can easily lead to thermal cracking.
The heat-affected zone (HAZ) in welding can easily lead to intergranular corrosion. This is because, within the HAZ, the base metal becomes chromium-depleted in the sensitization temperature range (450℃ to 850℃), making it difficult to passivate.
As a result, its corrosion resistance significantly decreases, and hence, it is preferentially corroded in the corresponding corrosive environment, widening the grain boundaries of the steel. At this point, the plasticity and strength of the corroded area have been severely compromised, leading to cracks and brittle fractures during cold bending, and a non-metallic sound when the corroded site hits the ground.
Stainless steel is a relatively corrosion-resistant type of steel, but it’s not absolutely rust-proof. To date, no steel has been invented that does not corrode under any conditions. Therefore, specific types of steel are designed for use in certain environments.
The corrosion resistance of steel increases with the content of chromium. When the chromium content reaches or exceeds 12%, the corrosion resistance of the steel changes dramatically, shifting from prone-to-rust to rust-resistant, and from non-corrosion-resistant to corrosion-resistant. Therefore, stainless steel is commonly referred to as an iron-based alloy with a chromium content of more than 12%.
The ability of steel to maintain its chemical stability (resistance to corrosion and scaling) at high temperatures is referred to as its thermal stability; the property of steel to have sufficient strength at high temperatures is called its thermal strength. Steel that possesses both thermal stability and thermal strength is known as heat-resistant steel.
1. Classification of Heat-Resistant Steel
(1) In pearlitic heat-resistant steel, the primary alloying elements are chromium, molybdenum, and vanadium, with their combined content typically less than 5%. This type is also referred to as low-alloy heat-resistant steel.
(2) Martensitic heat-resistant steel not only showcases high-temperature strength but also exhibits remarkable corrosion resistance. Both 1Cr13 and 2Cr13 steels can serve as heat-resistant steels as well as stainless steels.
(3) Ferritic Heat-resistant Steel
This type of steel has excellent resistance to high-temperature oxidation and corrosion, but it has poor heat strength and is prone to brittleness.
(4) Austenitic Heat-resistant Steel
Not only does this type of steel have high heat strength, but it also exhibits considerable plasticity, toughness, and excellent welding properties. Due to its single-phase austenitic structure, it also boasts superior corrosion resistance.
1. Weldability of Pearlitic Heat-Resistant Steel
The primary element in pearlitic heat-resistant steel is carbon, and it contains a certain amount of chromium and molybdenum. Some varieties also contain elements like vanadium, tungsten, silicon, titanium, and boron. The presence of these alloying elements makes the weld seam and heat-affected zone prone to hardening.
After welding, cooling in the air can easily produce hard and brittle martensite, which not only affects the mechanical properties of the welded joint but also generates significant internal stress. Combined with a high concentration of diffusing hydrogen, the weld seam and heat-affected zone are prone to cold cracking.
Furthermore, since pearlitic heat-resistant steel contains strong carbides like niobium, molybdenum, and chromium, and is typically used at high temperatures, it is susceptible to reheat cracking.
2. Weldability of Martensitic Heat-Resistant Steel
Martensitic heat-resistant steel primarily includes high-chromium steel with simple compositions, such as Cr13 and 2Cr13, as well as steel that adds alloy elements like Mo, V, W, Nb, etc., based on Chromium 12. These types of steel tend to undergo air quenching, resulting in poor weldability. After welding, they often form high-hardness martensite and a small amount of bainitic structures, leading to cold cracking.
3. Weldability of Ferritic Heat-Resistant Steel
Most ferritic heat-resistant steel is composed of high-chromium steel with w(Cr)>17% and a portion of Cr13-type steel. These types of steel do not undergo α→Y phase transformation during welding, showing no tendency to harden. However, the grains near the fusion line will rapidly enlarge, leading to the brittleness of the welded joint.
The higher the chromium content, and the longer the dwell time at high temperatures, the more severe the brittleness becomes. This brittleness cannot be refined through heat treatment, making it prone to cracks when welding rigid structures.
4. Weldability of Austenitic Heat-Resistant Steel
Austenitic heat-resistant steel has an austenitic microstructure as its matrix. This type of steel contains significant amounts of nickel, manganese, and nitrogen, which are austenite-forming elements. It possesses excellent high-temperature strength and structural stability above 600℃, combined with good welding performance. Hence, it is the most widely used type of heat-resistant steel in applications ranging from 600 to 1200℃.








